Abstract
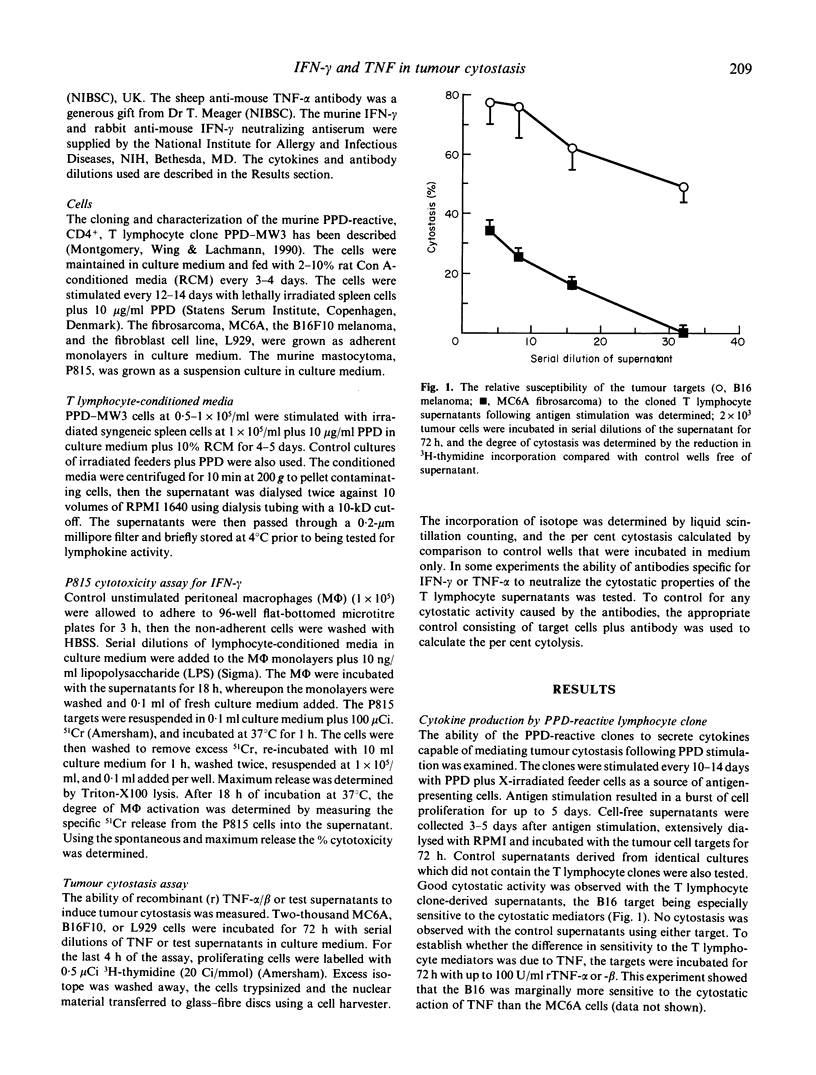
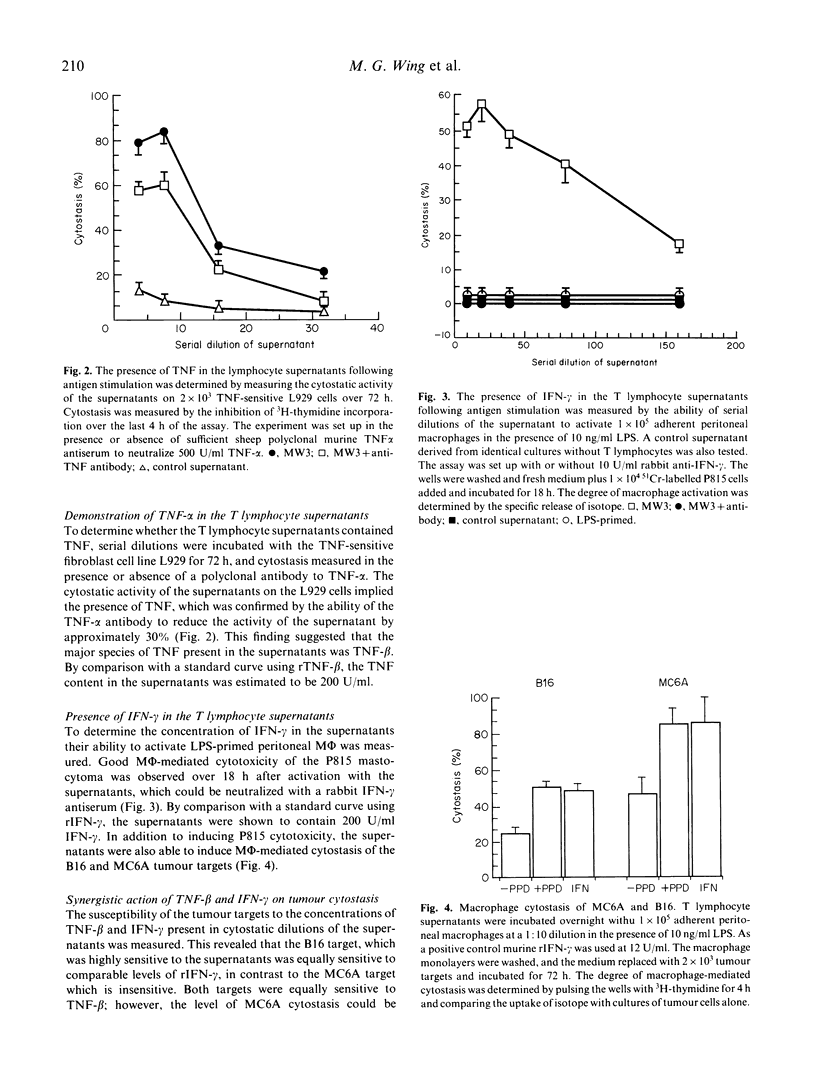
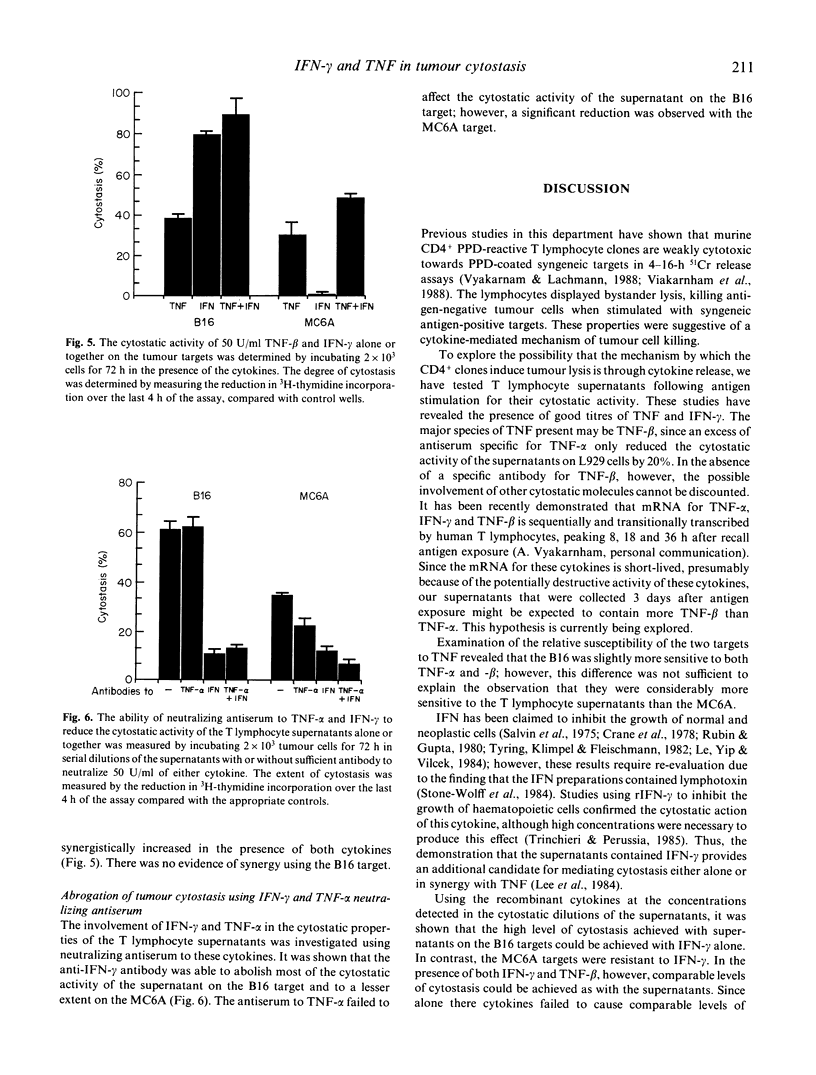
We have shown that a murine CD4+ PPD-reactive T lymphocyte clone was weakly cytotoxic towards the syngeneic tumour B16 melanoma and MC6A fibrosarcoma which had been coated with PPD using a monoclonal antibody-PPD heteroconjugate. Cell-free supernatants produced by PPD-stimulated T lymphocyte clones were however highly cytostatic for the two tumour targets when assayed over 48-72 h. In this study we have demonstrated good titres of tumour necrosis factor (TNF) and interferon-gamma (IFN-gamma) in the supernatants, which accounted for their observed cytostatic activity on the tumour targets. The high level of cytostasis seen with the B16 melanoma using the supernatants could be attributed to their sensitivity to the cytostatic activity of IFN-gamma; the lower levels of cytostasis seen with the IFN-gamma-resistant MC6A target was the result of IFN-gamma increasing the sensitivity of this target to TNF. Antibodies to IFN-gamma were able to neutralize the majority of the cytostatic activity of the supernatants on both targets, consistent with the role demonstrated for this lymphokine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Eessalu T. E., Hass P. E. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985 Dec 19;318(6047):665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Crane J. L., Jr, Glasgow L. A., Kern E. R., Youngner J. S. Inhibition of murine osteogenic sarcomas by treatment with type I or type II interferon. J Natl Cancer Inst. 1978 Sep;61(3):871–874. [PubMed] [Google Scholar]
- Hayashi H., Tanaka K., Jay F., Khoury G., Jay G. Modulation of the tumorigenicity of human adenovirus-12-transformed cells by interferon. Cell. 1985 Nov;43(1):263–267. doi: 10.1016/0092-8674(85)90031-5. [DOI] [PubMed] [Google Scholar]
- Le J., Prensky W., Yip Y. K., Chang Z., Hoffman T., Stevenson H. C., Balazs I., Sadlik J. R., Vilcek J. Activation of human monocyte cytotoxicity by natural and recombinant immune interferon. J Immunol. 1983 Dec;131(6):2821–2826. [PubMed] [Google Scholar]
- Le J., Yip Y. K., Vilcek J. Cytolytic activity of interferon-gamma and its synergism with 5-fluorouracil. Int J Cancer. 1984 Oct 15;34(4):495–500. doi: 10.1002/ijc.2910340411. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Aggarwal B. B., Rinderknecht E., Assisi F., Chiu H. The synergistic anti-proliferative effect of gamma-interferon and human lymphotoxin. J Immunol. 1984 Sep;133(3):1083–1086. [PubMed] [Google Scholar]
- Liu C. C., Steffen M., King F., Young J. D. Identification, isolation, and characterization of a novel cytotoxin in murine cytolytic lymphocytes. Cell. 1987 Nov 6;51(3):393–403. doi: 10.1016/0092-8674(87)90635-0. [DOI] [PubMed] [Google Scholar]
- Masson D., Nabholz M., Estrade C., Tschopp J. Granules of cytolytic T-lymphocytes contain two serine esterases. EMBO J. 1986 Jul;5(7):1595–1600. doi: 10.1002/j.1460-2075.1986.tb04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery A. M., Wing M. G., Lachmann P. J. A novel strategy for targeting CD4+ PPD-reactive T cells against tumour cells using PPD monoclonal antibody heteroconjugates. Clin Exp Immunol. 1990 Nov;82(2):200–207. doi: 10.1111/j.1365-2249.1990.tb05427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Pasternack M. S., Verret C. R., Liu M. A., Eisen H. N. Serine esterase in cytolytic T lymphocytes. Nature. 1986 Aug 21;322(6081):740–743. doi: 10.1038/322740a0. [DOI] [PubMed] [Google Scholar]
- Pfizenmaier K., Bartsch H., Scheurich P., Seliger B., Ucer U., Vehmeyer K., Nagel G. A. Differential gamma-interferon response of human colon carcinoma cells: inhibition of proliferation and modulation of immunogenicity as independent effects of gamma-interferon on tumor cell growth. Cancer Res. 1985 Aug;45(8):3503–3509. [PubMed] [Google Scholar]
- Rubin B. Y., Gupta S. L. Differential efficacies of human type I and type II interferons as antiviral and antiproliferative agents. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5928–5932. doi: 10.1073/pnas.77.10.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero V., Tavernier J., Fiers W., Baglioni C. Induction of the synthesis of tumor necrosis factor receptors by interferon-gamma. J Immunol. 1986 Apr 1;136(7):2445–2450. [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Nishio J., Neta R. Tumor suppression by a lymphokine released into the circulation of mice with delayed hypersensitivity. J Natl Cancer Inst. 1975 Nov;55(5):1233–1236. doi: 10.1093/jnci/55.5.1233. [DOI] [PubMed] [Google Scholar]
- Scheurich P., Krönke M., Schlüter C., Ucer U., Pfizenmaier K. Noncytocidal mechanisms of action of tumor necrosis factor-alpha on human tumor cells: enhancement of HLA gene expression synergistic with interferon-gamma. Immunobiology. 1986 Sep;172(3-5):291–300. doi: 10.1016/s0171-2985(86)80111-5. [DOI] [PubMed] [Google Scholar]
- Stone-Wolff D. S., Yip Y. K., Kelker H. C., Le J., Henriksen-Destefano D., Rubin B. Y., Rinderknecht E., Aggarwal B. B., Vilcek J. Interrelationships of human interferon-gamma with lymphotoxin and monocyte cytotoxin. J Exp Med. 1984 Mar 1;159(3):828–843. doi: 10.1084/jem.159.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyakarnam A., Lachmann P. J., Sia D. Y. The killing of tumour cell targets coupled to tuberculin (PPD) by human and murine PPD-reactive T helper clones. I. PPD specificity of killing. Scand J Immunol. 1988 Mar;27(3):337–346. doi: 10.1111/j.1365-3083.1988.tb02355.x. [DOI] [PubMed] [Google Scholar]
- Vyakarnam A., Lachmann P. J. The killing of tumour cell targets coupled to tuberculin (PPD) by human and murine PPD-reactive T helper clones. II. Major histocompatibility complex restriction of killing. Scand J Immunol. 1988 Mar;27(3):347–356. doi: 10.1111/j.1365-3083.1988.tb02356.x. [DOI] [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A. Cellular and humoral mechanisms of cytotoxicity: structural and functional analogies. Adv Immunol. 1987;41:269–332. doi: 10.1016/s0065-2776(08)60033-4. [DOI] [PubMed] [Google Scholar]


