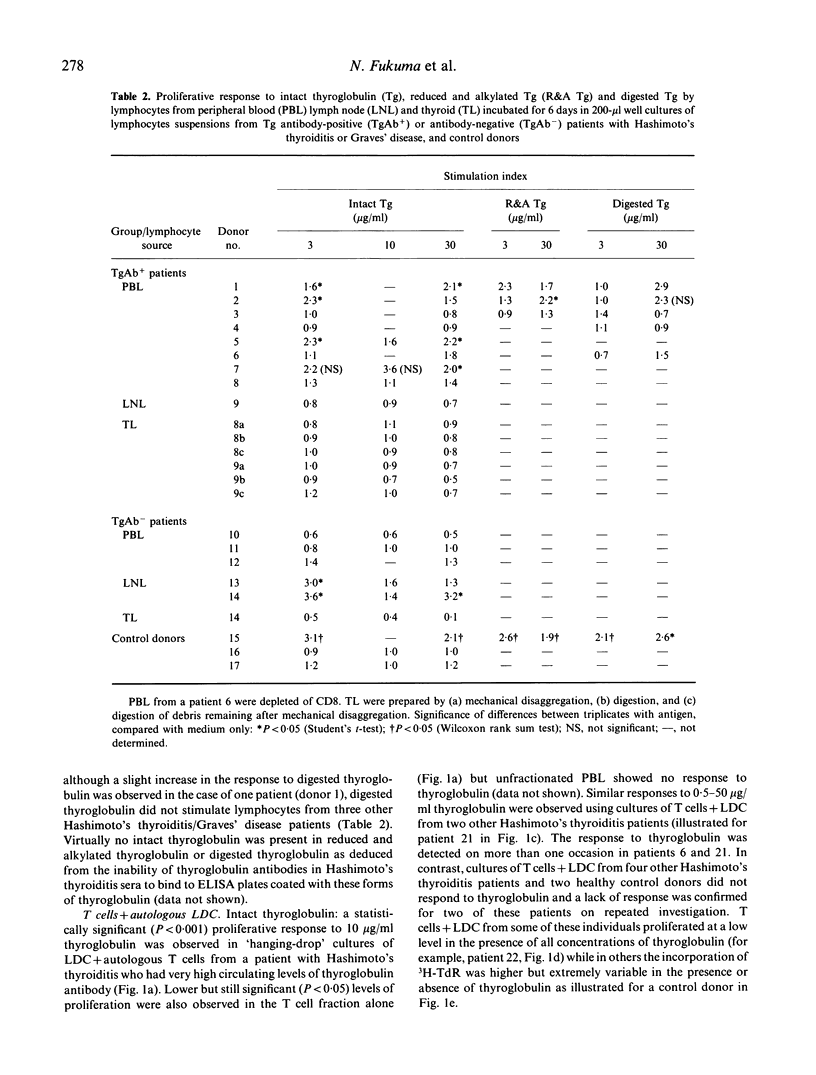
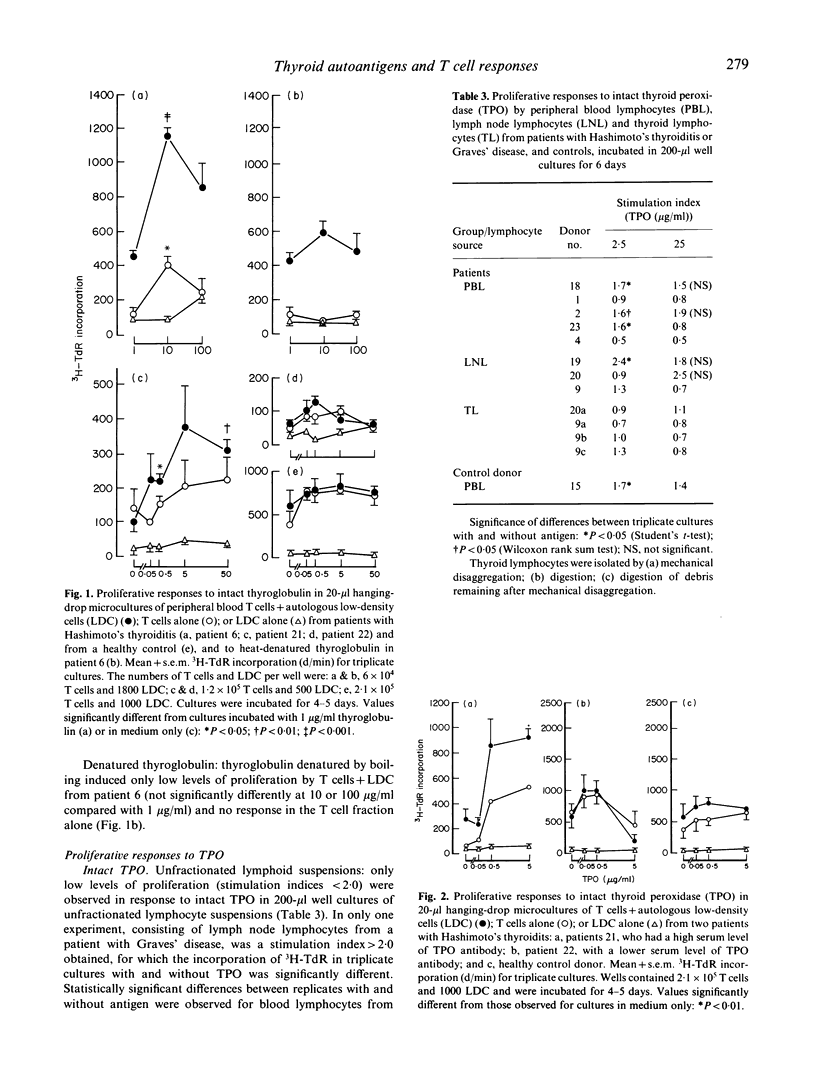
Abstract
We investigated the ability of T cells from patients with Hashimoto's thyroiditis and with Graves' disease as well as control donors to proliferate in response to thyroid peroxidase (TPO) and thyroglobulin using (i) lymphoid cells from different lymphoid organs; (ii) unfractionated or CD8- depleted lymphoid suspensions or T cells + autologous low density cells (LDC); (iii) 200-microliters well cultures and 20-microliters hanging-drop microcultures; and (iv) intact TPO and thyroglobulin, denatured thyroglobulin and 12 synthetic peptides predicted on the basis of the amino acid sequence of TPO to be T cell epitopes. In 200-microliters well cultures, proliferative responses (assessed in terms of 3H-thymidine uptake) to intact TPO or thyroglobulin, digested thyroglobulin or synthetic TPO peptides were not significantly different in unfractionated or CD8-depleted lymphoid suspensions from blood, thyroid or lymph nodes of TPO/thyroglobulin autoantibody-positive patients, autoantibody-negative patients or control donors. In contrast, blood T cells from some high titre patients with Hashimoto's thyroiditis (but not from healthy individuals) proliferated in response to intact thyroglobulin or TPO presented by autologous LDC in hanging-drop microcultures. Heat denatured thyroglobulin (with which thyroglobulin autoantibodies do not interact) did not stimulate proliferation and this observation, together with the ability of T cells from some patients to respond to intact thyroglobulin in the absence of LDC, indicated that thyroglobulin-specific B cells may be involved in antigen presentation. As we were unable to demonstrate proliferation by blood T cells + LDC from all thyroglobulin antibody-positive patients with Hashimoto's thyroiditis, our studies suggest that the presence of sufficient precursor T cells, as well as the number and type of antigen-presenting cells, are critical for T cell proliferative responses to human TPO and thyroglobulin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton M. C., McLachlan S. M., Pegg C. A., Dickinson A., Baylis P., Young E. T., Proctor S. J., Rees Smith B. Thyroid autoantibody synthesis by lymphocytes from different lymphoid organs: fractionation of B cells on density gradients. Immunology. 1985 Jun;55(2):271–279. [PMC free article] [PubMed] [Google Scholar]
- BOYSE E. A., OLD L. J., CHOUROULINKOV I. CYTOTOXIC TEST FOR DEMONSTRATION OF MOUSE ANTIBODY. Methods Med Res. 1964;10:39–47. [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Canonica G. W., Cosulich M. E., Croci R., Ferrini S., Bagnasco M., Dirienzo W., Ferrini O., Bargellesi A., Giordano G. Thyroglobulin-induced T-cell in vitro proliferation in Hashimoto's thyroiditis: identification of the responsive subset and effect of monoclonal antibodies directed to Ia antigens. Clin Immunol Immunopathol. 1984 Aug;32(2):132–141. doi: 10.1016/0090-1229(84)90115-6. [DOI] [PubMed] [Google Scholar]
- Czarnocka B., Ruf J., Ferrand M., Carayon P., Lissitzky S. Purification of the human thyroid peroxidase and its identification as the microsomal antigen involved in autoimmune thyroid diseases. FEBS Lett. 1985 Oct 7;190(1):147–152. doi: 10.1016/0014-5793(85)80446-4. [DOI] [PubMed] [Google Scholar]
- Farrant J., Bryant A. E., Chan J., Himsworth R. L. Thyroglobulin-treated blood dendritic cells induce IgG anti-thyroglobulin antibody in vitro in Hashimoto's thyroiditis. Clin Immunol Immunopathol. 1986 Dec;41(3):433–442. doi: 10.1016/0090-1229(86)90014-0. [DOI] [PubMed] [Google Scholar]
- Forouhi N. G., McLachlan S. M., Middleton S. L., Atherton M. C., Baylis P., Clark F., Smith B. R. T cell regulation of thyroglobulin autoantibody IgG subclasses in Hashimoto's thyroiditis. Clin Exp Immunol. 1987 Aug;69(2):314–322. [PMC free article] [PubMed] [Google Scholar]
- Harcourt G. C., Sommer N., Rothbard J., Willcox H. N., Newsom-Davis J. A juxta-membrane epitope on the human acetylcholine receptor recognized by T cells in myasthenia gravis. J Clin Invest. 1988 Oct;82(4):1295–1300. doi: 10.1172/JCI113729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabel P. J., Voorbij H. A., De Haan M., van der Gaag R. D., Drexhage H. A. Intrathyroidal dendritic cells. J Clin Endocrinol Metab. 1988 Jan;66(1):199–207. doi: 10.1210/jcem-66-1-199. [DOI] [PubMed] [Google Scholar]
- Katz D. R., Feldmann M., Tees R., Schreier M. H. Heterogeneity of accessory cells interacting with T-helper clones. Immunology. 1986 Jun;58(2):167–172. [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Kotani T., McBride O. W., Umeki K., Hirai K., Nakayama T., Ohtaki S. Human thyroid peroxidase: complete cDNA and protein sequence, chromosome mapping, and identification of two alternately spliced mRNAs. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5555–5559. doi: 10.1073/pnas.84.16.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. D., Katz D. R. Mechanisms of dendritic cell function. Immunol Today. 1990 Jun;11(6):206–211. doi: 10.1016/0167-5699(90)90084-m. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Bryant A., Edwards A. J., Burman S., Lever A., Clarke J., Webster A. D. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology. 1986 Apr;57(4):595–603. [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Chan J., Bryant A., Bedford P. A., Bateman C. Induction of autoimmunity with dendritic cells: studies on thyroiditis in mice. Clin Immunol Immunopathol. 1988 Sep;48(3):277–289. doi: 10.1016/0090-1229(88)90021-9. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Mertin J., Stackpoole A., Clark J. Induction of immune responses in vivo with small numbers of veiled (dendritic) cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6032–6035. doi: 10.1073/pnas.80.19.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Libert F., Ruel J., Ludgate M., Swillens S., Alexander N., Vassart G., Dinsart C. Complete nucleotide sequence of the human thyroperoxidase-microsomal antigen cDNA. Nucleic Acids Res. 1987 Aug 25;15(16):6735–6735. doi: 10.1093/nar/15.16.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie W. A., Schwartz A. E., Friedman E. W., Davies T. F. Intrathyroidal T cell clones from patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 1987 Apr;64(4):818–824. doi: 10.1210/jcem-64-4-818. [DOI] [PubMed] [Google Scholar]
- Magnusson R. P., Chazenbalk G. D., Gestautas J., Seto P., Filetti S., DeGroot L. J., Rapoport B. Molecular cloning of the complementary deoxyribonucleic acid for human thyroid peroxidase. Mol Endocrinol. 1987 Nov;1(11):856–861. doi: 10.1210/mend-1-11-856. [DOI] [PubMed] [Google Scholar]
- Male D. K., Champion B. R., Pryce G., Matthews H., Shepherd P. Antigenic determinants of human thyroglobulin differentiated using antigen fragments. Immunology. 1985 Mar;54(3):419–427. [PMC free article] [PubMed] [Google Scholar]
- McLachlan S. M., Clark S., Stimson W. H., Clark F., Smith B. R. Studies of thyroglobulin autoantibody synthesis using a micro-ELISA assay. Immunol Lett. 1982 Jan;4(1):27–33. doi: 10.1016/0165-2478(82)90073-6. [DOI] [PubMed] [Google Scholar]
- McLachlan S. M., Dickinson A., Baylis P., Proctor S., Rees Smith B. Enrichment and depletion of thyroglobulin autoantibody synthesizing lymphocytes. Clin Exp Immunol. 1983 Aug;53(2):397–405. [PMC free article] [PubMed] [Google Scholar]
- McLachlan S. M., Pegg C. A., Atherton M. C., Middleton S. L., Dickinson A., Clark F., Proctor S. J., Proud G., Rees Smith B. Subpopulations of thyroid autoantibody secreting lymphocytes in Graves' and Hashimoto thyroid glands. Clin Exp Immunol. 1986 Aug;65(2):319–328. [PMC free article] [PubMed] [Google Scholar]
- McLachlan S. M., Rapoport B. Evidence for a potential common T-cell epitope between human thyroid peroxidase and human thyroglobulin with implications for the pathogenesis of autoimmune thyroid disease. Autoimmunity. 1989;5(1-2):101–106. doi: 10.3109/08916938909029147. [DOI] [PubMed] [Google Scholar]
- Ohtaki S., Nakagawa H., Nakamura M., Yamazaki I. Reactions of purified hog thyroid peroxidase with H2O2, tyrosine, and methylmercaptoimidazole (goitrogen) in comparison with bovine lactoperoxidase. J Biol Chem. 1982 Jan 25;257(2):761–766. [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardt C. W., McLachlan S. M., Matheson J., Smith B. R. An enzyme-linked immunoassay for thyroid microsomal antibodies. J Immunol Methods. 1982 Dec 17;55(2):155–168. doi: 10.1016/0022-1759(82)90028-x. [DOI] [PubMed] [Google Scholar]
- Shimojo N., Saito K., Kohno Y., Sasaki N., Tarutani O., Nakajima H. Antigenic determinants on thyroglobulin: comparison of the reactivities of different thyroglobulin preparations with serum antibodies and T cells of patients with chronic thyroiditis. J Clin Endocrinol Metab. 1988 Apr;66(4):689–695. doi: 10.1210/jcem-66-4-689. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta L., Roques M., Torresani J., Rolland M., Lissitzky S. Human thyroglobulin. Physicochemical properties in relation to iodine content. Biochim Biophys Acta. 1968 Dec 3;168(3):507–521. doi: 10.1016/0005-2795(68)90184-0. [DOI] [PubMed] [Google Scholar]


