Abstract
Background
The Arabidopsis genome contains nine sucrose transporter paralogs falling into three clades: SUT1-like, SUT2 and SUT4. The carriers differ in their kinetic properties. Many transport proteins are known to exist as oligomers. The yeast-based split ubiquitin system can be used to analyze the ability of membrane proteins to interact.
Results
Promoter-GUS fusions were used to analyze the cellular expression of the three transporter genes in transgenic Arabidopsis plants. All three fusion genes are co-expressed in companion cells. Protein-protein interactions between Arabidopsis sucrose transporters were tested using the split ubiquitin system. Three paralogous sucrose transporters are capable of interacting as either homo- or heteromers. The interactions are specific, since a potassium channel and a glucose transporter did not show interaction with sucrose transporters. Also the biosynthetic and metabolizing enzymes, sucrose phosphate phosphatase and sucrose synthase, which were found to be at least in part bound to the plasma membrane, did not specifically interact with sucrose transporters.
Conclusions
The split-ubiquitin system provides a powerful tool to detect potential interactions between plant membrane proteins by heterologous expression in yeast, and can be used to screen for interactions with membrane proteins as baits. Like other membrane proteins, the Arabidopsis sucrose transporters are able to form oligomers. The biochemical approaches are required to confirm the in planta interaction.
Keywords: split-ubiquitin system, sucrose transporter, membrane protein, protein-protein interaction, regulation, companion cells
Background
Sucrose transport activity is essential for distribution of photoassimilates between photosynthetic tissues and tissues of high demand, including growing regions, storage compartments, and non-photosynthetic organs. Transport of sucrose from the apoplasm across the plasma membrane into sieve-elements or companion cells is mediated by sucrose transporters belonging to the major facilitator superfamily of membrane proteins. Members of the sucrose transporter family are characterized by twelve transmembrane spanning domains with cytosolic N- and C-termini [1] and a large central hydrophilic loop.
Arabidopsis contains nine sucrose transporter homologs (SUTs or SUCs) [2], several of which have been functionally characterized. AtSUC2 functions as a high affinity transporter expressed in companion cells [3-5]. The essential role of SUC2 in long distance transport was recently demonstrated by analysis of T-DNA insertional mutants into the AtSUC2 gene [6]. Export of sucrose from leaves was severely impaired even in heterozygous plants. In contrast, AtSUC1, another high-affinity sucrose transporter closely related to SUC2 [3,7] seems to be involved in pollen tube growth and anther dehiscence [8]. AtSUC5, which is 80% identical (amino acids) to AtSUC1, was isolated as a biotin transporter [9], but also transports sucrose with high affinity. In contrast, AtSUT4 serves as a low affinity transporter and is potentially involved in phloem loading in minor veins [10]. SUT2 was characterized as weak low affinity transporter, and based on circumstantial evidence it has been speculated that SUT2 may be a candidate for a postulated sucrose sensor fulfilling similar functions as the yeast sugar sensors SNF3 and RGT2 [11]. The other Arabidopsis genes are highly related to SUC1 and SUC2, indicating recent gene amplification events, but function of these genes is still not defined.
Rapid changes in sucrose transport activity together with coexistence of sucrose transport systems differing in their kinetic properties indicate that uptake and distribution of sucrose according to supply and demand within the plant is highly regulated. One efficient means of regulation of transport activity can also occur through oligomerization, as has been described for the animal glucose transporter GLUT1 [12,13], which constitutes a functional carrier regulated within a tetrameric complex. It would thus be interesting to understand the quaternary structure of sucrose transporters in Arabidopsis.
Analysis of mechanisms involved in regulation of transport processes across the plasma membrane is difficult, due to difficult accessibility of plasma membrane proteins to biochemical assays. Moreover, classical two-hybrid studies provide limited means of analysis, since most systems require targeting of the interacting proteins to the nucleus for reporter gene expression. The split ubiquitin system provides a new and powerful two-hybrid tool allowing the analysis of protein-protein interactions between membrane proteins [14-17]. The split-ubiquitin system had so far been used mainly to probe interactions of yeast proteins constituting the Sec62 ER protein translocation complex at the ER [14,18-21]22. However, little is known about the use of the split-ubiquitin system for detection of protein-protein interactions between heterologously expressed proteins. Two separately expressed parts of sucrose transporters were shown to reconstitute a functional sucrose transporter at the yeast plasma membrane [23], indicating that the split-ubiquitin system is suitable to study protein-protein interactions between different sucrose transporters.
The aim of this work was to analyze interactions between sucrose transporters and other integral membrane proteins, and membrane-associated soluble proteins. It could be shown that Arabidopsis sucrose transporters are co-expressed in companion cells and interact with themselves and with each other, while no interaction was observed with a variety of other plasma membrane proteins, soluble proteins of sucrose metabolism, or proteins of the endomembrane system, demonstrating that the split-ubiquitin system provides a powerful tool to detect specific interactions among plant membrane proteins by heterologous expression in yeast.
Results
Co-expression of Arabiopsis sucrose transporters in companion cells
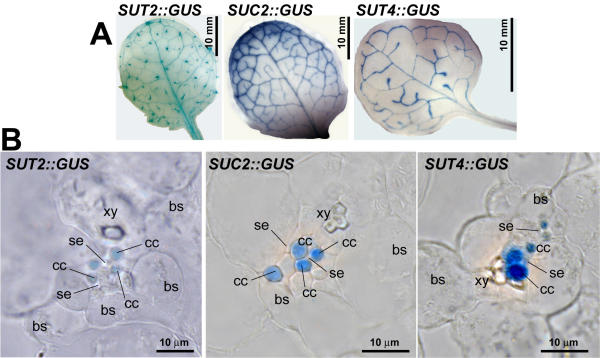
Promoter-GUS fusions were used to determine the expression pattern of the three sucrose transporters histochemically. The expression pattern of the high affinity AtSUC2 [4] and the low affinity AtSUT4 [10], and the putative sucrose sensor AtSUT2 [11] overlaps in minor veins of leaves of mature plants (Fig. 1A). AtSUC2::GUS was expressed in companion cells, consistent with previous immunolocalization studies [5]. This pattern coincides with weak expression of AtSUT2::GUS and strong expression of AtSUT4::GUS in the same cell type (Fig. 1B). Although differences in expression pattern of the three genes become apparent during the development of the plant, the clear overlap of expression of three transporters in the same cell type may have significant function during specific stages of development. However, it cannot be excluded, that the RNA expression pattern as derived from promoter-reporter studies may not entirely reflect the in-vivo protein localization pattern. The expression of SUT2 in companion cells is in disagreement with immunolocalization data in Arabidopsis [24].
Figure 1.
Overlapping expression of sucrose transporters in Arabidopsis. (A) GUS-expression mediated by promoter fragments of AtSUC2, AtSUT2, and AtSUT4 in leaves of Arabidopsis. (B) Cellular localization of GUS expression in minor veins of leaves embedded in LRwhite on 5 μm sections.
Generation of split-ubiquitin fusion constructs
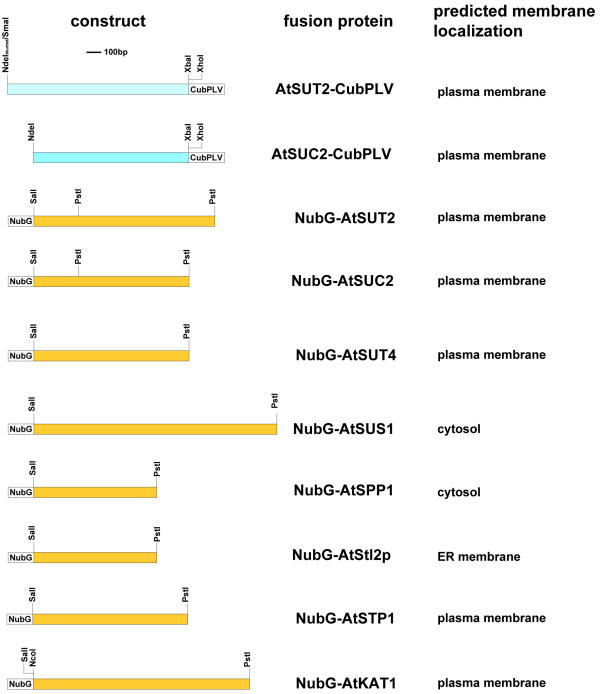
To study the interaction between the co-expressed Arabidopsis sucrose transporters, two transporters, i.e. SUT2 and SUC2, were fused with the C-terminal half of ubiquitin and the artificial transcription factor PLV (Fig. 2) as bait constructs. SUT2, SUC2, and SUT4 were fused with a mutated form of the N-terminal half of ubiquitin, NubG (Fig. 2) as prey constructs. A series of NubG-fusion proteins including membrane proteins of different compartments such as a the plasma membrane potassium channel AtKAT1 [25], the plasma membrane monosaccharide transporter AtSTP1 [26], a SEC12 homolog (AtStl2p) as an ER membrane protein [27], and orthologs of the soluble, but also partially membrane-bound sucrose metabolizing enzymes sucrose phosphate phosphatase [28] and sucrose synthase [29], was used to probe the specificity of the interactions and possibly detect interactions between sucrose transporters and enzymes of sucrose metabolism (Fig. 2).
Figure 2.
Constructs for split ubiquitin system. Two sucrose transporters (AtSUT2, AtSUC2) were cloned as CubPLV-fusion proteins, sucrose transporters (AtSUT2, AtSUC2, AtSUT4) and a variety of membrane proteins (AtSTP1, AtKAT1, AtSt12p), as well as cytosolic proteins (AtSUS1, AtSPP1) were cloned as NubG-fusion proteins. The predicted membrane location is indicated for each protein.
Split-ubiquitin analysis of sucrose transporter interactions
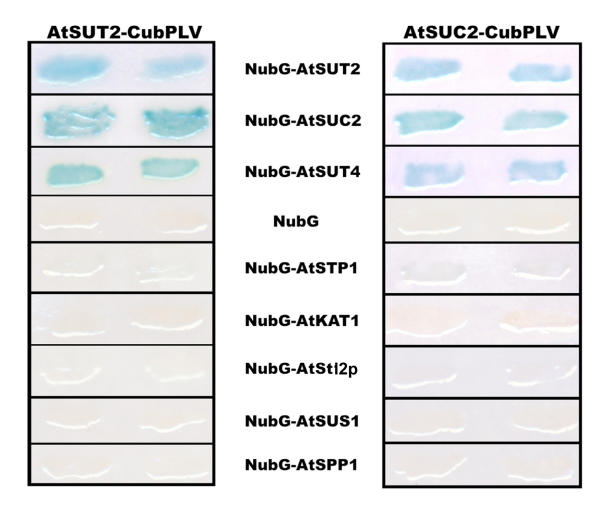
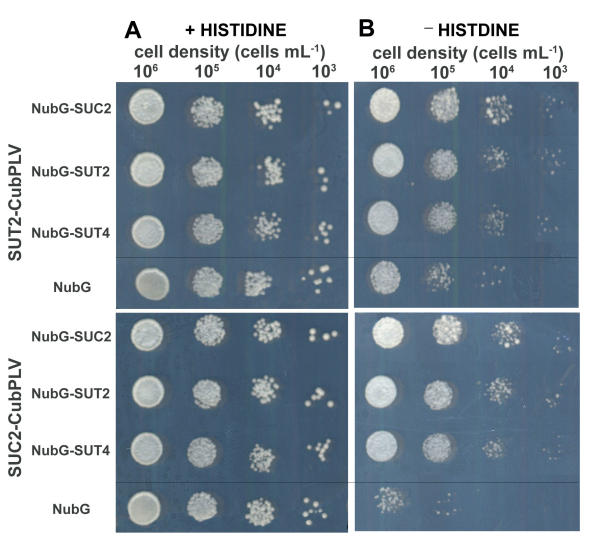
The bait constructs were stably integrated into the genome of the reporter yeast strain. The transformed bait strains were subsequently transformed with the prey constructs. Both AtSUT2 as well as AtSUC2 were able to interact with themselves and with NubG-fusions of sucrose transporters AtSUT2, AtSUC2, and AtSUT4, but not with soluble NubG alone (Fig. 3). The results indicate that the three proteins can form oligomers. Interactions between sucrose transporters were confirmed using growth of the auxotrophic strain in absence of histidine. On control plates with histidine, yeast cells bearing the bait constructs AtSUC2-CubPLV or AtSUT2-CubPLV in combination with sucrose transporter prey constructs grow equally well as cells bearing bait constructs in combination with NubG only (Fig. 4A). In contrast, on plates without histidine, growth of yeast cells bearing interacting sucrose transporters is significantly stronger compared to cells bearing bait proteins in combination with NubG only (Fig. 4B). Thus, both markers detecting cleavage of the artificial transcription factor from the CubPLV upon protein-protein interaction can be used to study interactions between two membrane proteins.
Figure 3.
Interactions of SUT2 and SUC2 as baits with other sucrose transporters detected by the split-ubiquitin system using LacZ as a reporter gene. Interaction of SUT2-CubPLV and SUC2-CubPLV as a bait was tested against various NubG-fusions of membrane proteins and soluble proteins as prey. Positive interaction was visualized by β-galactosidase expression in filter assays.
Figure 4.
Protein-protein interactions determined by the split ubiquitin system using HIS3 as reporter gene. (A) Growth of yeast cells on control plates with histidine. (B) Growth of yeast cells on plates without histidine demonstrating interaction of AtSUT2 and AtSUC2 as baits with other sucrose transporters as prey, and no growth of cells expressing bait constructs and NubG. Cells were spotted on SD medium with adenine and with or without histidine in 10 μL at cell densities indicated.
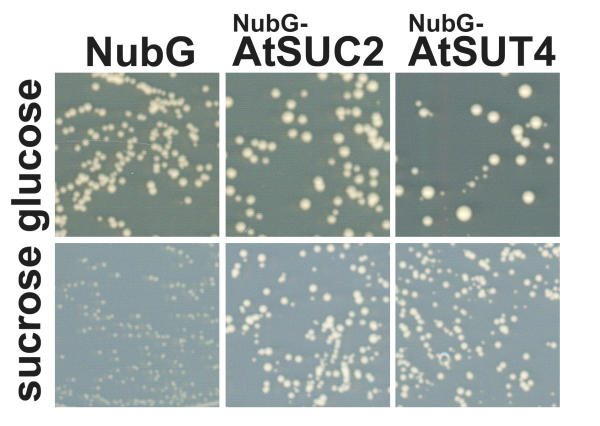
To confirm the targeting of the NubG-fusion proteins to the plasma membrane and to show their functionality, NubG-AtSUC2 and NubG-AtSUT4 were tested for their ability to complement growth of the yeast strain SUSY7/ura3 on sucrose [11,30]. In both cases, the N-terminal fusion of NubG to the sucrose transporters did not impair their transport function, as demonstrated by the growth of SUSY7/ura3 containing the respective fusion proteins on sucrose as the sole carbon source (Fig. 5). Kinetic analysis (data not shown) revealed no significant difference between sucrose affinity of NubG-fusion proteins and published sucrose affinities [3,10].
Figure 5.
Functionality of NubG-AtSUC2 and NubG-AtSUT4 fusion proteins. Growth of yeast strain SUSY7/ura3 bearing respective fusion proteins or the empty NubG-vector on medium containing 2% glucose or 2% sucrose as the sole carbon source after four days of growth at 28°C.
To exclude non-specific interactions due to random collisions in the plasma membrane, interactions were tested between control baits. No interactions were observed with the glucose transporter AtSTP1 and the potassium channel AtKAT1 [23,31], demonstrating specificity of the interaction between the three different sucrose transporters (Fig. 3,4). Furthermore, no interaction was found between AtSUT2 and AtSUC2 and the ER-protein AtStl2p excluding non-specific interactions within the endomembrane system during plasma membrane targeting (Fig. 3). The results further support the potential of the Arabidopsis sucrose transporters to form homo- or hetero-oligomers.
Since soluble enzymes involved in sucrose metabolism, such as sucrose synthase and sucrose-6-phosphate phosphatase were found to be membrane-associated [32,33], sucrose may also be channeled directly from transport to metabolism by an interaction of sucrose transporters with key enzymes of sucrose metabolism, similarly to glucose in tight associations of hexokinase with glucose transport [34-36]. Therefore sucrose synthase and sucrose phosphate phosphatase were used as NubG fusions as prey and tested against the different sucrose transporter baits (Fig. 3). No interaction was found between sucrose transporters AtSUT2 or AtSUC2 and sucrose synthase (AtSUS1) or sucrose-6-phosphate phosphatase (AtSPP1).
Discussion
The potential role of homomerization of sucrose transporters
Interactions detected between sucrose transporters provide first indications that sucrose transporters may exist as homodimers and possibly also as heterodimers or even higher order oligomeric structures. Dimerization may not be necessary for function per se, as concluded from studies on lactose permease, which is functional also as a monomer [37]. However, dimerization may contribute to protein stability or may have a role in targeting to the plasma membrane. For glucose transporters, it is proposed that native structure is established before translocation of the transporters to the plasma membrane [38]. Dimerization and tetramerization of mammalian glucose transporters was shown to be important for regulation of transport properties. Binding of substrate to one transport site changes the substrate affinity of the interacting partner in a cooperative manner [13]. This dynamic change in transport capacity is highly relevant for glucose homeostasis in mammalian cells. In addition, regulation of glucose transport is modulated by oligomerization, since tetrameric but not dimeric GLUT1 is subject to direct regulation by cytosolic ATP [39]. In addition, the stabilization of tetrameric states by disulfide bridges between subunits is dependent on the redox-status of the cellular environment [12,38]. Oligomerization is also required for proton co-transport of the bacterial lactose transporter LacS [40], thus the function as homomer may be valid for transporters in general. Sucrose transport is a highly regulated process, which needs to be highly dynamic over a wide range of conditions. Therefore, dynamic regulation of sucrose transport through formation or stabilization of homo-oligomers may allow fast adaptation of transport to changing conditions.
The potential role of heteromeric sucrose transporter complexes
The co-expressed sucrose transporters AtSUC2, AtSUT2 and AtSUT4 showed the potential of heteromeric interactions. So far, heteromeric associations were not observed for mammalian glucose transporters [41]. However, identification of a larger number of paralogues suggests also the possibility that GLUTs can form hetero-oligomers in certain cell types. The dramatic dominant negative effect observed in a yeast mutant GSF4-1 on overall glucose transport activity, due to a fusion event between HXT1 and HXT4, may be explained by the formation of inactive complexes suppressing activity of many other members of the yeast HXT family. This provides indirect evidence that also in yeast related transporters form hetero-oligomers [42].
The formation of heteromeric structures allows even more dynamic regulation of sucrose transport, especially if heteromers consist of subunits with different substrate affinities, as for example the high affinity AtSUC2 and the low affinity AtSUT4. Considering the hypothesized function of AtSUT2 as a sucrose sensor [11], one can envisage a model in which a putative sucrose sensor can directly modulate the transport activity of an interacting sucrose transporter through cooperative effects. It became obvious from analyses in yeast, that certain micro-environments of the plasma membrane, the so called lipid rafts, can change in composition due to intra- or extracellular stimuli [43], which in turn may favor certain protein-protein interactions resulting in the activation of signaling cascades or transport processes. The typical property of lipid rafts is their stability in the detergent Triton X-100. Thus, a more detailed analysis of the in planta biochemistry of interacting sucrose transporters is necessary involving the analysis of Triton X-100 extraction to detect such higher order homo- or heteromeric complexes.
Although co-expression of the three transporters in companion cells of Arabidopsis suggests a tight regulatory interaction between these transporters, the interaction between sucrose transporters detected by the split-ubiquitin system has to be validated in planta, before a full understanding of sucrose transporter regulation is possible. Given the potential of sucrose transporters to form homo- and heteromers, the observed overlapping but also distinct expression pattern of the three different sucrose transporters may reflect different regions of phloem function.
Conclusions
In conclusion, this work demonstrates that protein-protein interactions among plasma membrane proteins can be detected using the split ubiquitin system. Using a variety of membrane proteins as well as cytosolic proteins as controls, the interactions between plant sucrose transporters were shown to be specific for the family of Arabidopsis sucrose transporters, suggesting a general function of MFS transport proteins in higher order complexes similar to mammalian glucose transporters [13]. The split-ubiquitin system is a valuable tool to characterize these interactions in specific and quantitative manner. Moreover, the split-ubiquitin system may serve as tool to screen for interactions with membrane proteins as bait in order to identify interactions with unknown partners.
Methods
Split-ubiquitin constructs
A modified split-ubiquitin system [14] was used to investigate protein-protein interactions between AtSUT2, or AtSUC2 as baits, and other Arabidopsis proteins as prey in the yeast strain L40 (MATa ade3 trp1 leu2 his3 LYS2::lexA-HIS3 URA3::lexA-lacZ). Interactions between membrane proteins was monitored by interaction of two membrane bound fusion proteins leading to release of an artificial transcription factor consisting of Protein A, LexA, and VP16 (PLV) and inducing transcription of reporter genes (LacZ, HIS3). The bait cDNAs were cloned in frame with the C-terminal (Cub) subdomain of ubiquitin followed by PLV. Prey cDNAs were fused to the N-terminal (NubG) subdomain of ubiquitin. Fusion vectors have been described [23] and can be obtained from Igor Stagliar, Institute of Veterinary Biochemistry, University of Zürich. Interaction between "bait" and "prey" fusion proteins leads to cleavage of PLV by ubiquitin-specific proteases, and subsequent activation of reporter genes. The integrative bait vector pY-CubPLV contains the wheat germ agglutinin-binding protein WBP1-promoter, allowing constitutive, but low expression of bait fusion protein. AtSUT2 (At2g02860) and AtSUC2 (At1g22710) were cloned as fusion proteins with Cub using restriction sites NdeI and XhoI (Fig. 1). After linearization with ClaI, bait-constructs were integrated into LEU2 of strain L40 using lithium-acetate based transformation [44]. AtSUT2, AtSUC2, AtSUT4 (At1g09960), AtSTP1 (At1g11260), AtSPP1 (sucrose-phosphate phosphatase; At2g35840), and AtSUS1 (sucrose synthase; At3g43190), and the ER-protein AtSt12p (At5g50550) were amplified by RT-PCR, sequenced and cloned as NubG-fusion proteins under control of the ADH1-promoter using the restriction sites SalI and PstI, or NcoI, PstI for NubG-fusion with AtKAT1 (At5g46240) (Fig. 1).
Yeast complementation
Functionality of the sucrose transporter-NubG-fusions was tested using yeast strain SUSY7/ura3 [11,30] transformed with plasmids bearing respective fusion constructs. To test for growth on sucrose, three colonies from each transformation were resuspended in 5 mL water and 50 μL aliquots were plated both on yeast minimal medium supplemented with tryptophan and either glucose (2%), or sucrose (2%, pH 4). Plates were incubated for 3 (glucose) and 6 days (sucrose) at 28°C. Sucrose was obtained from Carl Roth, Karlsruhe, Germany)
β-galactosidase assay
β-galactosidase activity was determined by filter assays [45]. Cells were streaked on reinforced nitrocellulose membranes, placed on SD agar plates containing adenine and histidine and grown for two days. Filters were removed from the agar, frozen in liquid nitrogen, placed on filter paper saturated with Z-buffer (60 mM Na2PO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4 pH 7.0) containing 35 mM β-mercaptoethanol and 1.5 mg mL-1 5-bromo-4-chloro-3-indolyl-3-D-galactoside and incubated at 37°C for two hours.
Promoter GUS analysis
A 2.1 kb fragment of the AtSUC2 promoter was cloned into pGPTV-hpt [46] using SalI and SmaI. AtSUT2::GUS [11], AtSUT4::GUS [10], and AtSUC2::GUS expression was analyzed in mature plants grown in soil and seedlings grown on 6% sucrose in the dark as described [11] in presence of 3 mM K-ferricyanide an 0.5 mM K-ferrocyanide at pH 7. Leaves of 15-day old plants were fixed in 4% glutaraldehyde, dehydrated in ethanol and embedded in LRWhite and 5 μm sections were prepared [31]. For each gene, several plants and leaves of different age were analyzed, representative photographs are shown.
Authors' contributions
WS and AR carried out the molecular genetic studies, SL participated in the design of the study. JMW and WBF conceived of the study, and participated in its design and coordination. WS and WBF drafted the manuscript All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Petr Obrdlik for critical reading of the manuscript. We gratefully acknowledge support by grants from Deutsche Forschungsgemeinschaft (SFB446), the EU project No QLG2-CT-2001-01297 „Associoport" and the Gottfried Wilhelm Leibniz award to WBF. WS was supported by a fellowship from the Deutsche Studienstiftung.
Contributor Information
Waltraud X Schulze, Email: waltraud@bmb.sdu.dk.
Anke Reinders, Email: anke@tc.umn.edu.
John Ward, Email: jward@tc.umn.edu.
Sylvie Lalonde, Email: sylvie.lalonde@zmbp.uni-tuebingen.de.
Wolf B Frommer, Email: frommer@zmbp.uni-tuebingen.de.
References
- Stolz J, Ludwig A, Stadler R, Biesgen C, Hagemann K, Sauer N. Structural analysis of a plant sucrose carrier using monoclonal antibodies and bacteriophage lambda surface display. FEBS Letters. 1999;453:375–379. doi: 10.1016/S0014-5793(99)00756-5. [DOI] [PubMed] [Google Scholar]
- Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, Willmitzer L. Metabolite profiling for plant functional genomics. Nature Biotechnology. 2000;18:1157–1161. doi: 10.1038/81137. [DOI] [PubMed] [Google Scholar]
- Sauer N, Stolz J. SUC1 and SUC2: Two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker's yeast and identification of the histidine-tagged protein. The Plant Journal. 1994;6:67–77. doi: 10.1046/j.1365-313X.1994.6010067.x. [DOI] [PubMed] [Google Scholar]
- Truernit E, Sauer N. The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of beta-glucuronidase to the phloem: Evidence for phloem loading and unloading by SUC2. Planta. 1995;196:564–570. doi: 10.1007/BF00203657. [DOI] [PubMed] [Google Scholar]
- Stadler R, Sauer N. The Arabidopsis thaliana AtSUC2 gene is specifically expressed in companion cells. Botanica Acta. 1996;109:299–306. [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proceedings of the National Academy of Sciences of the USA. 2000;97:13979–13984. doi: 10.1073/pnas.250473797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JJ, Theodoulou F, Sauer N, Sanders D, Miller AJ. A kinetic model with ordered cytoplasmic dissociation for SUC1, an Arabidopsis H+/sucrose cotransporter expressed in Xenopus oocytes. Journal of Membrane Biology. 1997;159:113–125. doi: 10.1007/s002329900275. [DOI] [PubMed] [Google Scholar]
- Stadler R, Truernit E, Gahrtz M, Sauer N. The AtSUC1 sucrose carrier may represent the osmotic driving force for anther dehiscence and pollen tube growth in Arabidopsis. The Plant Journal. 1999;19:269–278. doi: 10.1046/j.1365-313X.1999.00527.x. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Stolz J, Sauer N. Plant sucrose-H+ symporters mediate the transport of vitamin H. The Plant Journal. 2000;24:503–509. doi: 10.1046/j.1365-313x.2000.00900.x. [DOI] [PubMed] [Google Scholar]
- Weise A, Barker L, Kühn C, Lalonde S, Buschmann H, Frommer WB, Ward JM. A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell. 2000;12:1345–1355. doi: 10.1105/tpc.12.8.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, Hellmann H, Schulze W, Ward JM, Frommer WB. SUT2, a putative sucrose sensor in sieve elements. Plant Cell. 2000;12:1153–1164. doi: 10.1105/tpc.12.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottola RJ, Cloherty EK, Coderre PE, Hansen A, Hebert DN, Carruthers A. Glucose transporter function is controlled by transporter oligomeric structure. A single, intramolecular disulfide promoter GLUT1 tetramerization. Biochemistry. 1995;34:9734–9747. doi: 10.1021/bi00030a011. [DOI] [PubMed] [Google Scholar]
- Hamill S, Cloherty EK, Carruthers A. The human erythrocyte sugar transporter presents two sugar import sites. Biochemistry. 1999;38:16974–16983. doi: 10.1021/bi9918792. [DOI] [PubMed] [Google Scholar]
- Stagljar I, Korostensky C, Johnsson N, Te Heesen S. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proceedings of the National Academy of Sciences of the USA. 1998;95:5187–5192. doi: 10.1073/pnas.95.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely BT, Rossi FMV, Tillotson B, Palmer M, Estelles A, Blau HM. Epidermal growth factor receptor dimerization monitored in live cells. Nature Biotechnology. 2000;18:218–222. doi: 10.1038/72686. [DOI] [PubMed] [Google Scholar]
- Stagljar I, te Heesen S. Detecting interactions between membrane proteins in vivo using chimeras. Methods in Enzymology. 2000;327:190–198. doi: 10.1016/s0076-6879(00)27276-5. [DOI] [PubMed] [Google Scholar]
- Ehrhard KN, Jacoby JJ, Fu X-Y, Jahn R, Dohlman HG. Use of G-protein fusions to monitor integral membrane protein-protein interactions in yeast. Nature Biotechnology. 2000;18:1075–1080. doi: 10.1038/80274. [DOI] [PubMed] [Google Scholar]
- Raquet X, Eckert JH, Müller S, Johnsson N. Detection of altered protein conformations in living cells. Journal of Molecular Biology. 2001;305:927–938. doi: 10.1006/jmbi.2000.4239. [DOI] [PubMed] [Google Scholar]
- Dünnwald M, Varshavsky A, Johnsson N. Detection of transient in vivo interactions between substrate and transporter during protein translocation into the endoplasmic reticulum. Molecular Biology of the Cell. 1999;10:329–344. doi: 10.1091/mbc.10.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laser H, Bongards C, Schüller J, Heck S, Johnsson N, Leming N. A new screen for protein interactions reveals that the Saccharomyces cerevisiae high mobility group proteins NhpA/B are involved in the regulation of the GAL1 promoter. Proceedings of the National Academy of Sciences of the USA. 2000;97:13732–13737. doi: 10.1073/pnas.250400997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittke S, Lewke N, Müller S, Johnsson N. Probing the molecular environment of membrane proteins in vivo. Molecular Biology of the Cell. 1999;10:2519–2530. doi: 10.1091/mbc.10.8.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N, Varshavsky A. Split-ubiquitin as a sensor of protein interactions in vivo. Proceedings of the National Academy of Sciences of the USA. 1994;91:10340–10344. doi: 10.1073/pnas.91.22.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders A, Schulze W, Thaminy S, Stagljar I, Frommer WB, Ward JM. Intra- and intermolecular interactions in sucrose transporters at the plasma membrane detected by the split-ubiquitin system and functional assays. Structure. 2002;10:763–772. doi: 10.1016/S0969-2126(02)00773-6. [DOI] [PubMed] [Google Scholar]
- Meyer S, Melzer M, Truernit E, Hummer C, Besenbeck R, Stadler R, Sauer N. AtSUC3, a gene encoding a new Arabidopsis sucrose transporter, is expressed in cells adjacent to the vascular tissue and in a carpel cell layer. The Plant Journal. 2000;24:869–882. doi: 10.1046/j.1365-313x.2000.00934.x. [DOI] [PubMed] [Google Scholar]
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N, Friedländer K, Gräml-Wicke U. Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. The EMBO Journal. 1990;9:3045–3050. doi: 10.1002/j.1460-2075.1990.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert C, Gensse M, Gaillardin C. Fission yeast and a plant have functional homologues of the Sar1 and Sec12 proteins involved in ER to Golgi traffic in budding yeast. The EMBO Journal. 1992;11:4205–4211. doi: 10.1002/j.1460-2075.1992.tb05514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE, Ashton AR, Hatch MD, Heldt HW. Purification, molecular cloning, and sequence analysis of sucrose-6F-phosphate phosphohydrolase from plants. Proceedings of the National Academy of Sciences of the USA. 2000;97:12914–12919. doi: 10.1073/pnas.230430197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, Frommer WB, Salanoubat M, Willmitzer L. Expression of an Arabidopsis sucrose synthase gene indicates a role in metabolization of sucrose both during phloem loading and in sink organs. The Plant Journal. 1993;4:367–377. doi: 10.1046/j.1365-313X.1993.04020367.x. [DOI] [PubMed] [Google Scholar]
- Lemoine R. Sucrose transporters in plants: update on function and structure. Biochimica et Biophysica Acta Biomembranes. 2000;1465:246–262. doi: 10.1016/S0005-2736(00)00142-5. [DOI] [PubMed] [Google Scholar]
- Reinders A, Schulze W, Kühn C, Barker L, Ward JM, Frommer WB. Protein-protein interactions between sucrose transporters of different affinities co-localized in the same enucleate sieve element. The Plant Cell. 2002;14:1567–1577. doi: 10.1105/tpc.002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Huber SC. Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Critical Reviews in Biochemistry and Molecular Biology. 2000;35:253–289. doi: 10.1080/10409230008984165. [DOI] [PubMed] [Google Scholar]
- Lunn JE, Ashtion AR, Hatch MD, Heldt HW. Purification, molecular cloning, and sequence analysis of sucrose-6F-phosphate phosphohydrolase from plants. Proceedings of the National Academy of Sciences of the USA. 2000;97:12914–12919. doi: 10.1073/pnas.230430197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan S, Johnston M. Function and regulation of yeast hexose transporters. Microbiology and Molecular Biology Reviews. 1999;63:554–569. doi: 10.1128/mmbr.63.3.554-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis AJ, Sui D, Riedel KD, Hofmann NR, Moss SB, Wilson JE, Kopf GS. A novel NH2-terminal, nonhydrophobic motif targets a male germ cell-specific hexokinase to the endoplasmatic reticulum and plasma membrane. Journal of Biological Chemistry. 1999;274:34467–34475. doi: 10.1074/jbc.274.48.34467. [DOI] [PubMed] [Google Scholar]
- Wiese A, Gröner F, Sonnewald U, Deppner H, Lerchl J, Hebbeker U, Flügge UI, Weber A. Spinach hexokinase I is located in the outer envelope membrane of plastids. FEBS Letters. 1999;461:13–18. doi: 10.1016/S0014-5793(99)01417-9. [DOI] [PubMed] [Google Scholar]
- Sahin-Tóth M, Lawrence MC, Kaback HR. Properties of permease dimer, a fusion protein containing two lactose permease molecules from Escherichia coli. Proceedings of the National Academy of Sciences of the USA. 1994;91:5421–5425. doi: 10.1073/pnas.91.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Carruthers A. Glucose transporter oligomeric structure determines transporter function. Reversible redox-dependent interconversions of tetrameric and dimeric GLUT1. Journal of Biological Chemistry. 1992;267:23829–23838. [PubMed] [Google Scholar]
- Levine KB, Cloherty EK, Fidyk NJ, Carruthers A. Structural and physiologic determinants of human erythrocyte sugar transport regulation by adenosine triphosphate. Biochemistry. 1998;37:12221–12232. doi: 10.1021/bi980585y. [DOI] [PubMed] [Google Scholar]
- Veenhoff LM, Heuberger EHML, Poolman B. The lactose transport protein is a cooperative dimer with two sugar translocation pathways. The EMBO Journal. 2001;20:3056–3062. doi: 10.1093/emboj/20.12.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Carruthers A. Cholate-solubilized erytrhocyte glucose transporters exist as a mixture of homodimers and homotetramers. Biochemistry. 1991;30:4654–4658. doi: 10.1021/bi00233a003. [DOI] [PubMed] [Google Scholar]
- Sherwood PW, Katic I, Sanz P, Carlson M. A glucose transporter chimera confers a dominant negative glucose starvation phenotype in Saccharomyces cerevisiae. Genetics. 2000;155:989–992. doi: 10.1093/genetics/155.2.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nature Reviews Molecular Cell Biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Gietz D, St Jean A, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Research. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer SW, Elledge SJ, Davis RW. Detection of altered protein conformations in living cells. Proceedings of the National Academy of Sciences of the USA. 1992;89:11589–11593. doi: 10.1073/pnas.89.23.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Kemper E, Schell J, Masterson R. New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Molecular Biology. 1992;20:1195–1197. doi: 10.1007/BF00028908. [DOI] [PubMed] [Google Scholar]