Abstract
Neonatal B cells have been considered immature because of their impaired capacity to produce immunoglobulins in response to polyclonal activators in vitro. Here we demonstrate that cord blood mononuclear cells (MNC) produce normal levels of IgE in vitro when cultured in the presence of interleukin-4 (IL-4), indicating that the B cells are mature in their capacity to switch to IgE-producing cells. However, in contrast to adult peripheral blood T cells, cord blood T cells failed to produce detectable levels of IL-4 upon activation by phytohaemagglutinin (PHA) concanavalin A (Con A) or combinations of PHA and the phorbol ester TPA. Interferon-gamma (IFN-gamma) production by cord blood T cells following activation by Con A or PHA was also strongly reduced. However, high levels of IFN-gamma, significantly higher than those produced by adult T cells, were synthesized in response to combinations of PHA and TPA, indicating that IFN-gamma production by cord blood T cells is not intrinsically defective. In contrast, cord blood T cells produced levels of IL-2 that were significantly higher than those obtained by adult T cells tested in parallel. Collectively, our data indicate that the minimal levels of IgE production measured in cord blood (less than 1 U/ml) are not due to immaturity of the cord blood B cells, but may be associated with the failure of cord blood T cells to produce detectable levels of IL-4, which has been shown to be responsible for induction of IgE synthesis both in vitro and in vivo.
Full text
PDF
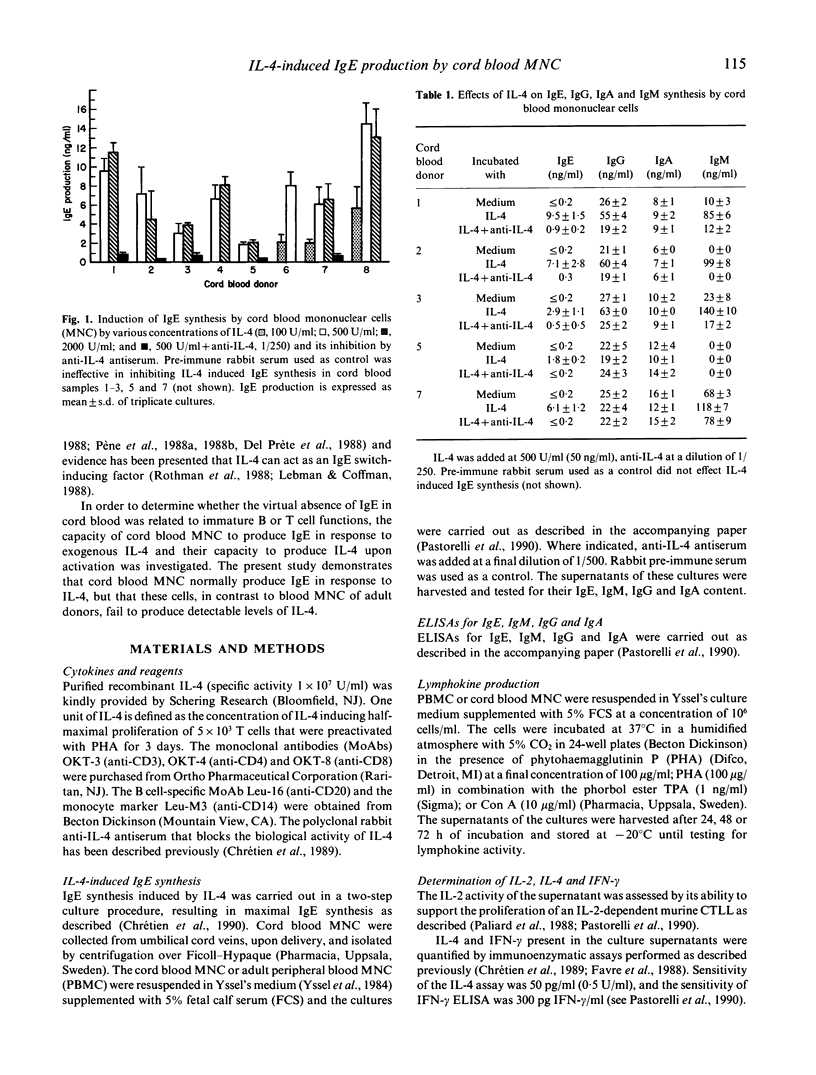
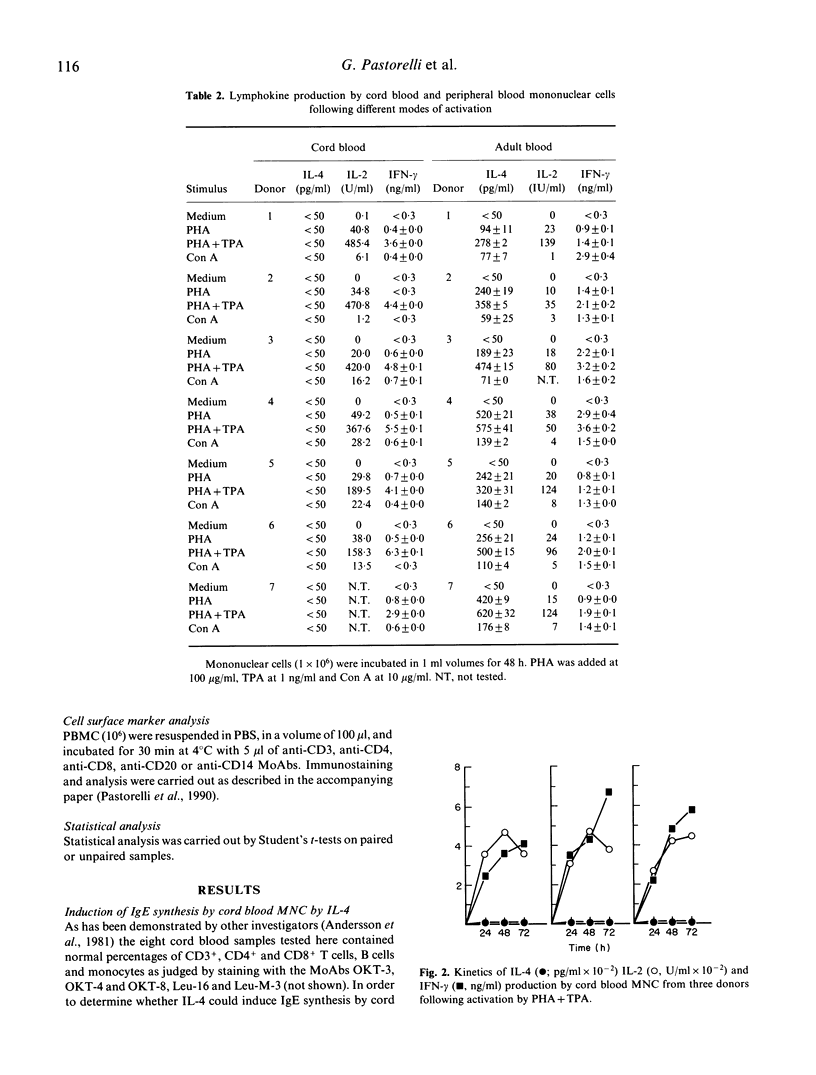
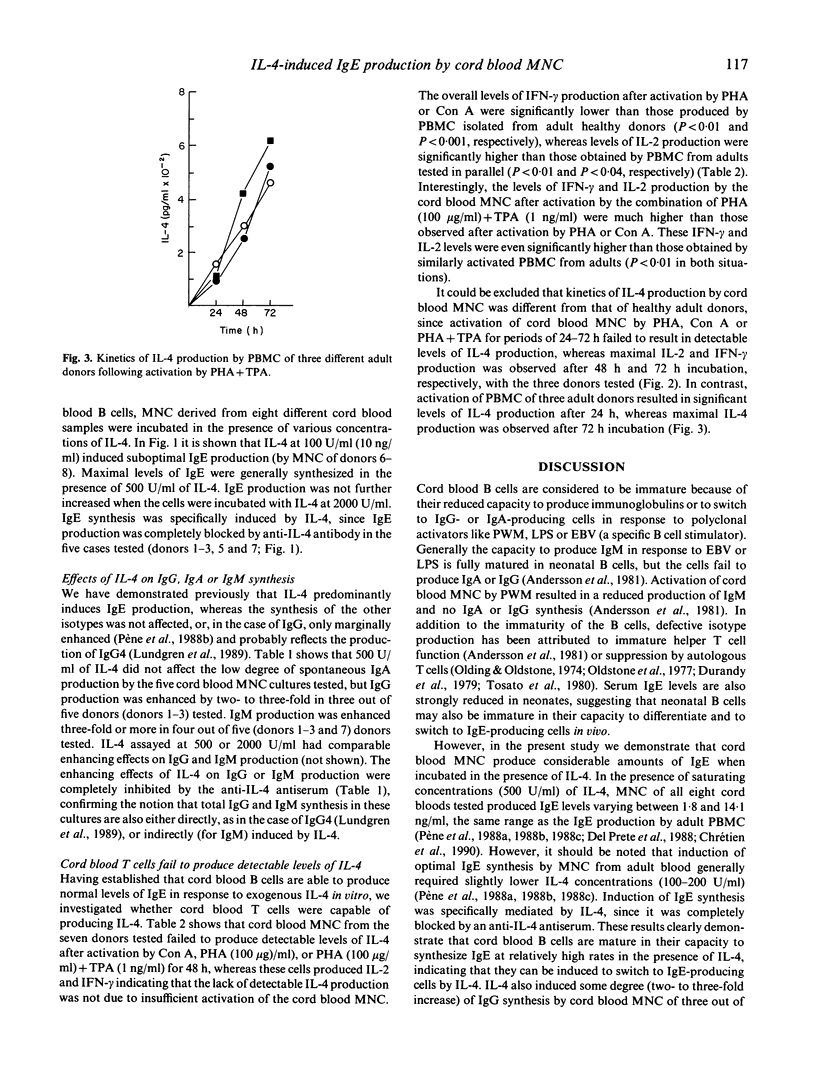


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H., Cartwright B. B., Sell S. H. Ontogeny of human cell-mediated immunity: age-related variation of in vitro infantile lymphocyte transformation. Infect Immun. 1976 Apr;13(4):1170–1175. doi: 10.1128/iai.13.4.1170-1175.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B., Skoglund A. C., Rönnholm M., Lindsten T., Lamon E. W., Collisson E. W., Walia A. S. Functional aspects of IgM and IgG Fc receptors on murine T lymphocytes. Immunol Rev. 1981;56:5–50. doi: 10.1111/j.1600-065x.1981.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Baley J. E., Schacter B. Z. Mechanisms of diminished natural killer cell activity in pregnant women and neonates. J Immunol. 1985 May;134(5):3042–3048. [PubMed] [Google Scholar]
- Bryson Y. J., Winter H. S., Gard S. E., Fischer T. J., Stiehm E. R. Deficiency of immune interferon production by leukocytes of normal newborns. Cell Immunol. 1980 Sep 15;55(1):191–200. doi: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- Campbell A. C., Waller C., Wood J., Aynsley-Green A., Yu V. Lymphocyte subpopulations in the blood of newborn infants. Clin Exp Immunol. 1974 Dec;18(4):469–482. [PMC free article] [PubMed] [Google Scholar]
- Chrétien I., Pène J., Brière F., De Waal Malefijt R., Rousset F., De Vries J. E. Regulation of human IgE synthesis. I. Human IgE synthesis in vitro is determined by the reciprocal antagonistic effects of interleukin 4 and interferon-gamma. Eur J Immunol. 1990 Feb;20(2):243–251. doi: 10.1002/eji.1830200203. [DOI] [PubMed] [Google Scholar]
- Chrétien I., Van Kimmenade A., Pearce M. K., Banchereau J., Abrams J. S. Development of polyclonal and monoclonal antibodies for immunoassay and neutralization of human interleukin-4. J Immunol Methods. 1989 Feb 8;117(1):67–81. doi: 10.1016/0022-1759(89)90120-8. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Del Prete G., Maggi E., Parronchi P., Chrétien I., Tiri A., Macchia D., Ricci M., Banchereau J., De Vries J., Romagnani S. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988 Jun 15;140(12):4193–4198. [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Active suppression of B lymphocyte maturation by two different newborn T lymphocyte subsets. J Immunol. 1979 Dec;123(6):2644–2650. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Holmes J., Ohara J., Tung A. S., Sample J. V., Paul W. E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988 Oct 1;141(7):2335–2341. [PubMed] [Google Scholar]
- Glaisher I. L. Smallpox vaccination. Can Med Assoc J. 1969 Oct 4;101(7):119–119. [PMC free article] [PubMed] [Google Scholar]
- Granberg C., Hirvonen T. Cell-mediated lympholysis by fetal and neonatal lymphocytes in sheep and man. Cell Immunol. 1980 Apr;51(1):13–22. doi: 10.1016/0008-8749(80)90233-6. [DOI] [PubMed] [Google Scholar]
- Granberg C., Manninen K., Toivanen P. Cell-mediated lympholysis by human neonatal lymphocytes. Clin Immunol Immunopathol. 1976 Sep;6(2):256–263. doi: 10.1016/0090-1229(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Kurnick J. Newborn T cell suppression: early appearance, maintenance in culture, and lack of growth factor suppression. J Immunol. 1981 Jan;126(1):50–53. [PubMed] [Google Scholar]
- Lebman D. A., Coffman R. L. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988 Sep 1;168(3):853–862. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren M., Persson U., Larsson P., Magnusson C., Smith C. I., Hammarström L., Severinson E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol. 1989 Jul;19(7):1311–1315. doi: 10.1002/eji.1830190724. [DOI] [PubMed] [Google Scholar]
- Olding L. B., Oldstone M. B. Lymphocytes from human newborns abrogate mitosis of their mother's lymphocytes. Nature. 1974 May 10;249(453):161–162. doi: 10.1038/249161a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Moretta L. Active thymus derived suppressor lymphocytes in human cord blood. Nature. 1977 Sep 22;269(5626):333–335. doi: 10.1038/269333a0. [DOI] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Pastorelli G., Roncarolo M. G., Peronne C., Tovo P. A., de Vries J. E. The capacity of interleukin-4 to induce in vitro IgE synthesis by B cells of patients with common variable immunodeficiency. Clin Exp Immunol. 1990 Oct;82(1):120–127. doi: 10.1111/j.1365-2249.1990.tb05414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pène J., Chrétien I., Rousset F., Brière F., Bonnefoy J. Y., de Vries J. E. Modulation of IL-4-induced human IgE production in vitro by IFN-gamma and IL-5: the role of soluble CD23 (s-CD23). J Cell Biochem. 1989 Mar;39(3):253–264. doi: 10.1002/jcb.240390305. [DOI] [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Bonnefoy J. Y., Spits H., Yokota T., Arai N., Arai K., Banchereau J. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6880–6884. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Paliard X., Banchereau J., Spits H., De Vries J. E. IgE production by normal human B cells induced by alloreactive T cell clones is mediated by IL-4 and suppressed by IFN-gamma. J Immunol. 1988 Aug 15;141(4):1218–1224. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Wideman J., Bonnefoy J. Y., De Vries J. E. Interleukin 5 enhances interleukin 4-induced IgE production by normal human B cells. The role of soluble CD23 antigen. Eur J Immunol. 1988 Jun;18(6):929–935. doi: 10.1002/eji.1830180615. [DOI] [PubMed] [Google Scholar]
- Rayfield L. S., Brent L., Rodeck C. H. Development of cell-mediated lympholysis in human foetal blood lymphocytes. Clin Exp Immunol. 1980 Dec;42(3):561–570. [PMC free article] [PubMed] [Google Scholar]
- Stites D. P., Carr M. C., Fudenberg H. H. Ontogeny of cellular immunity in the human fetus: development of responses to phytohemagglutinin and to allogeneic cells. Cell Immunol. 1974 Mar 30;11(1-3):257–271. doi: 10.1016/0008-8749(74)90026-4. [DOI] [PubMed] [Google Scholar]
- Taylor S., Bryson Y. J. Impaired production of gamma-interferon by newborn cells in vitro is due to a functionally immature macrophage. J Immunol. 1985 Mar;134(3):1493–1497. [PubMed] [Google Scholar]
- Wakasugi N., Virelizier J. L., Arenzana-Seisdedos F., Rothhut B., Huerta J. M., Russo-Marie F., Fiers W. Defective IFN-gamma production in the human neonate. II. Role of increased sensitivity to the suppressive effects of prostaglandin E. J Immunol. 1985 Jan;134(1):172–176. [PubMed] [Google Scholar]
- Wakasugi N., Virelizier J. L. Defective IFN-gamma production in the human neonate. I. Dysregulation rather than intrinsic abnormality. J Immunol. 1985 Jan;134(1):167–171. [PubMed] [Google Scholar]
- Weston W. L., Carson B. S., Barkin R. M., Slater G. D., Dustin R. D., Hecht S. K. Monocyte-macrophage function in the newborn. Am J Dis Child. 1977 Nov;131(11):1241–1242. doi: 10.1001/archpedi.1977.02120240059011. [DOI] [PubMed] [Google Scholar]
- Yssel H., De Vries J. E., Koken M., Van Blitterswijk W., Spits H. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984 Aug 3;72(1):219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]



