Abstract
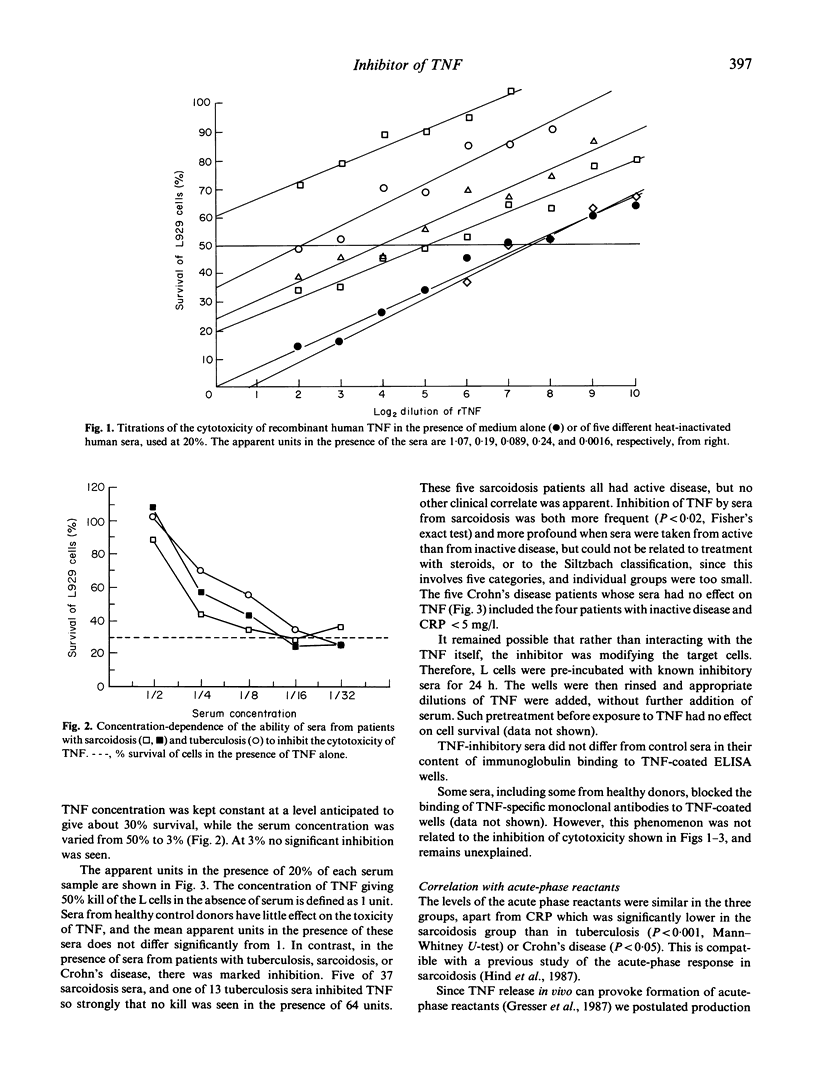
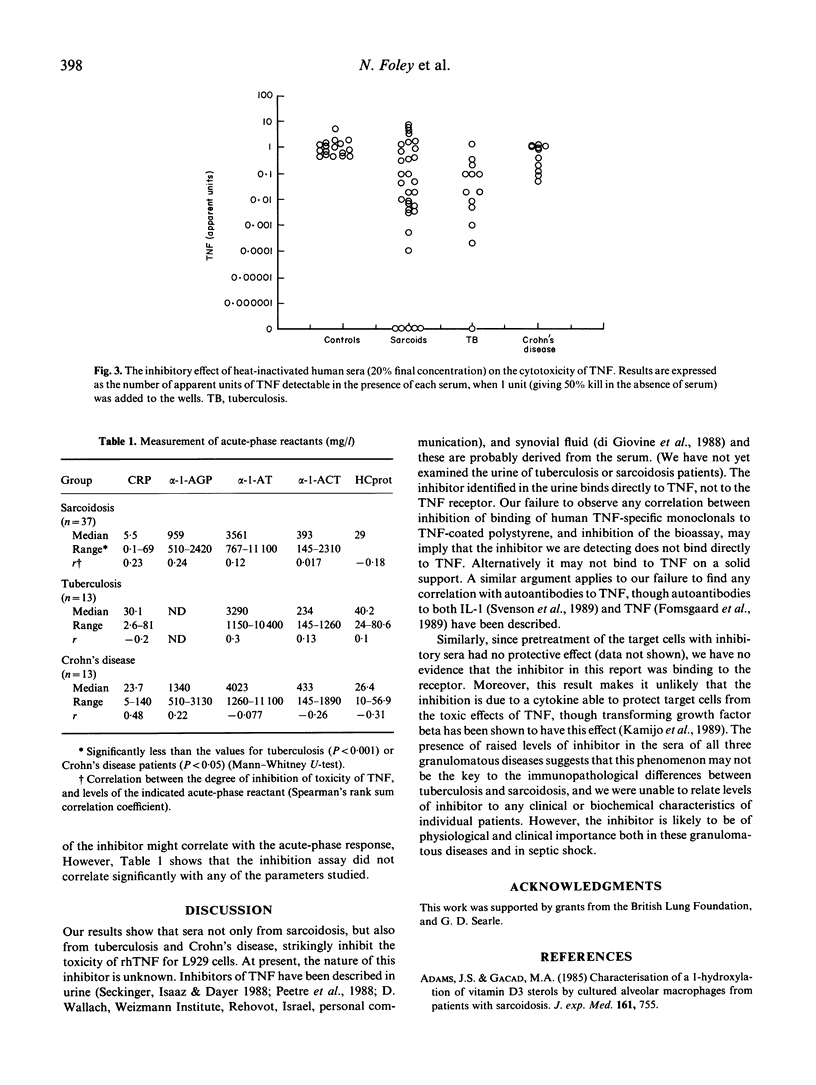
The activated macrophages present in the T cell-dependent granulomata of sarcoidosis and tuberculosis are primed for enhanced release of cytokines including tumour necrosis factor (TNF or cachectin). Release of this cytokine can induce an acute-phase response, fever, and necrosis in suitably prepared sites of inflammation; if chronic, its presence may contribute to weight loss. These clinical features are characteristic of tuberculosis, but not of sarcoidosis, though alveolar macrophages from both diseases release large quantities of TNF in vitro. We therefore postulated the presence in sarcoidosis patients of an inhibitor of TNF. We have studied levels of TNF inhibitory activity by determining the quantity of TNF required to give 50% kill of L929 cells in the presence of 20% heat-inactivated serum derived from various disease states (37 sarcoidosis, 13 tuberculosis, 13 Crohn's disease, 17 healthy donors). Normal sera used in this way do not inhibit significantly, but inhibition of TNF toxicity is caused by most sera from both sarcoidosis and tuberculosis. Used at 20%, five out of 37 sarcoidosis sera and one out of 13 tuberculosis sera caused complete inhibition of TNF, even when the latter was added at 100 times the concentration required to give 50% kill in control wells. This inhibitor may have an important physiological role.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. S., Gacad M. A. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med. 1985 Apr 1;161(4):755–765. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachwich P. R., Lynch J. P., 3rd, Larrick J., Spengler M., Kunkel S. L. Tumor necrosis factor production by human sarcoid alveolar macrophages. Am J Pathol. 1986 Dec;125(3):421–425. [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomsgaard A., Svenson M., Bendtzen K. Auto-antibodies to tumour necrosis factor alpha in healthy humans and patients with inflammatory diseases and gram-negative bacterial infections. Scand J Immunol. 1989 Aug;30(2):219–223. doi: 10.1111/j.1365-3083.1989.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Gresser I., Delers F., Tran Quangs N., Marion S., Engler R., Maury C., Soria C., Soria J., Fiers W., Tavernier J. Tumor necrosis factor induces acute phase proteins in rats. J Biol Regul Homeost Agents. 1987 Oct-Dec;1(4):173–176. [PubMed] [Google Scholar]
- Hind C. R., Flint K. C., Hudspith B. N., Felmingham D., Brostoff J., Johnson N. M. Serum C-reactive protein concentrations in patients with pulmonary sarcoidosis. Thorax. 1987 May;42(5):332–335. doi: 10.1136/thx.42.5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo R., Takeda K., Nagumo M., Konno K. Suppression of TNF-stimulated proliferation of diploid fibroblasts and TNF-induced cytotoxicity against transformed fibroblasts by TGF-beta. Biochem Biophys Res Commun. 1989 Jan 16;158(1):155–162. doi: 10.1016/s0006-291x(89)80191-3. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Reichel H., Bishop J. E., Norman A. W. gamma-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun. 1985 Mar 15;127(2):596–603. doi: 10.1016/s0006-291x(85)80202-3. [DOI] [PubMed] [Google Scholar]
- Meager A., Parti S., Leung H., Peil E., Mahon B. Preparation and characterization of monoclonal antibodies directed against antigenic determinants of recombinant human tumour necrosis factor (rTNF). Hybridoma. 1987 Jun;6(3):305–311. doi: 10.1089/hyb.1987.6.305. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedwin G. E., Svedersky L. P., Bringman T. S., Palladino M. A., Jr, Goeddel D. V. Effect of interleukin 2, interferon-gamma, and mitogens on the production of tumor necrosis factors alpha and beta. J Immunol. 1985 Oct;135(4):2492–2497. [PubMed] [Google Scholar]
- Peetre C., Thysell H., Grubb A., Olsson I. A tumor necrosis factor binding protein is present in human biological fluids. Eur J Haematol. 1988 Nov;41(5):414–419. doi: 10.1111/j.1600-0609.1988.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Attiyah R. A., Foley N. The role of cytokines in the immunopathology of tuberculosis, and the regulation of agalactosyl IgG. Lymphokine Res. 1989 Fall;8(3):323–328. [PubMed] [Google Scholar]
- Rook G. A. Role of activated macrophages in the immunopathology of tuberculosis. Br Med Bull. 1988 Jul;44(3):611–623. doi: 10.1093/oxfordjournals.bmb.a072271. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Fraher L., Barker S., Karmali R., O'Riordan J., Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986 Jan;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A. The role of vitamin D in tuberculosis. Am Rev Respir Dis. 1988 Oct;138(4):768–770. doi: 10.1164/ajrccm/138.4.768. [DOI] [PubMed] [Google Scholar]
- Rothstein J. L., Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci U S A. 1988 Jan;85(2):607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckinger P., Isaaz S., Dayer J. M. A human inhibitor of tumor necrosis factor alpha. J Exp Med. 1988 Apr 1;167(4):1511–1516. doi: 10.1084/jem.167.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W., Jr, Senterfitt V. C. Endotoxin-induced hepatic damage in BCG-infected mice. Am J Pathol. 1972 Apr;67(1):23–40. [PMC free article] [PubMed] [Google Scholar]
- Svenson M., Poulsen L. K., Fomsgaard A., Bendtzen K. IgG autoantibodies against interleukin 1 alpha in sera of normal individuals. Scand J Immunol. 1989 Apr;29(4):489–492. doi: 10.1111/j.1365-3083.1989.tb01149.x. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]


