Abstract
We identify and characterize a gene cluster in El Tor Vibrio cholerae that encodes a cytotoxic activity for HEp-2 cells in vitro. This gene cluster contains four genes and is physically linked to the cholera toxin (CTX) element in the V. cholerae genome. We demonstrate by using insertional mutagenesis that this gene cluster is required for the cytotoxic activity. The toxin, RtxA, resembles members of the RTX (repeats in toxin) toxin family in that it contains a GD-rich repeated motif. Like other RTX toxins, its activity depends on an activator, RtxC, and an associated ABC transporter system, RtxB and RtxD. In V. cholerae strains of the classical biotype, a deletion within the gene cluster removes rtxC and eliminates cytotoxic activity. Other strains, including those of the current cholera pandemic, contain a functional gene cluster and display cytotoxic activity. Thus, the RTX gene cluster in El Tor O1 and O139 strains might have contributed significantly to their emergence. Furthermore, the RTX toxin of V. cholerae may be associated with residual adverse properties displayed by certain live, attenuated cholera vaccines.
Toxigenic strains of the Gram-negative bacterium Vibrio cholerae cause a life-threatening diarrheal disease that can kill its victims within hours of the onset of symptoms. The disease is endemic in the Indian subcontinent and continues to reemerge elsewhere in Asia, Africa, and the Americas with the incidence estimated to exceed 5 million cases each year (1–3). Although over 180 serogroups of V. cholerae exist, only the O1 and O139 serovars are known to cause epidemic cholera. The O1 serogroup can be divided further into two biotypes, classical and El Tor, based on various biochemical and phenotypic differences (4). Since 1817, seven cholera pandemics have occurred, of which the classical biotype is responsible for at least the fifth (1881–1896) and the sixth (1899–1923). The El Tor biotype is responsible for the seventh and current pandemic (1961-present). Toxigenic strains of O139 serovar appeared in India and Bangladesh in late 1992 as the first non-O1 serovar to cause epidemic cholera (5, 6). These strains are closely related and probably derived from toxigenic El Tor O1 strains (7). The factors that allowed El Tor biotype and O139 serotype to overtake the classical strains and establish pandemics remain a mystery.
Work focusing on the pathogenesis of V. cholerae led to the identification of several key virulence factors, including the cholera toxin responsible for the diarrhea and the toxin-coregulated pilus essential for colonizing the human small intestine (8, 9). Several groups tried to construct live, attenuated V. cholerae vaccines by deleting the genes for cholera toxin and several other putative accessory toxins (10–16). However, many of these strains remain reactogenic in human subjects, causing diarrhea or other significant symptoms (17). The virulence factors responsible for the residual reactogenicity remain to be identified.
Bacterial genomics offers an opportunity to identify undiscovered potential virulence determinants based on their sequence similarity to virulence genes of other pathogenic microorganisms. We report here the discovery of a V. cholerae toxin gene cluster that was uncovered by a combination of genomic sequence analysis, genetic mapping, and representational difference analysis. The toxin belongs to the RTX (repeat in toxin) family of toxins, a group of related exotoxins produced by a variety of pathogenic Gram-negative bacteria with hemolytic, leukotoxic, and leukocyte-stimulating activities (18, 19). We report the determination of the genomic organization of the V. cholerae RTX toxin gene cluster and the demonstration that these genes’ products are responsible for the cytotoxic activity observed when V. cholerae bacteria are added to mammalian cells in vitro.
MATERIALS AND METHODS
Chemicals, Enzymes, Bacterial Strains, and Plasmids.
All chemicals were obtained from Sigma, and all enzymes were purchased from New England Biolabs. Bacterial strains and plasmids used are listed in Table 1. Escherichia coli and V. cholerae were grown using conditions described (20).
Table 1.
Assay for cytotoxic activity of V. cholerae strains
| Strain | Source (biotype)/Genotype | RTX | CTX | TLC | Toxicity |
|---|---|---|---|---|---|
| N16961 | Bangladesh patient, 1975 (E) | + | + | + | + |
| KFV42 | N16961 Δhap | + | + | + | + |
| P27459 | Bangladesh patient, 1976 (E) | + | + | + | + |
| E7946 | Bahrain patient, 1978 (E) | + | + | + | + |
| C6709 | Peru patient, 1991 (E) | + | + | + | + |
| MO10 | India patient, 1992 | + | + | + | + |
| Bang-1 | P27459 Δcore | + | − | + | + |
| Bah-1 | E7946 Δcore | + | − | + | + |
| Peru-1 | C6709 Δcore | + | − | + | + |
| Bengal-1 | MO10 Δcore | + | − | + | + |
| Bang-2 | P27459 ΔattRS1 | − | − | + | − |
| Bah-2 | E7946 ΔattRS1 | − | − | + | − |
| Peru-2 | C6709 ΔattRS1 | − | − | + | − |
| Bengal-2 | MO10 ΔattRS1 | − | − | + | − |
| WLV12 | N16961 rtxA∷pWL127 | − | + | + | − |
| WLV13 | P27459 rtxA∷pWL127 | − | + | + | − |
| WLV14 | E7946 rtxA∷pWL127 | − | + | + | − |
| WLV15 | C6709 rtxA∷pWL127 | − | + | + | − |
| WLV16 | MO10 rtxA∷pWL127 | − | + | + | − |
| JAS6 | N16961 rtxC∷pJAS6 | − | + | + | − |
| O395 | India patient, 1964 (C) | − | + | + | − |
| 569B | India patient, 1948 (C) | − | + | + | − |
The V. cholerae biotype is listed in parenthesis: classical (C) or El Tor (E). MO10 is a V. cholerae 0139 strain. Δcore is a deletion of the CTX element except the RS element (45). ΔattRS1 is a deletion involving the two HindIII sites bracketing the CTX element (10, 12, 16). Southern analysis of DNA prepared from the indicated strains or PCR with approporiate primers was used to detect rtxA or rtxC, ctxAB, and the TLC element. Cytotoxicity was scored visually in a blind assay.
Recombinant DNA Methods.
Recombinant DNA methods were performed according to standard protocols (21, 22). Sequencing was performed on an ABI 373A sequencer (Applied Biosystems), and sequences were assembled with macvector software (Oxford Molecular Group). Nucleotide and derived sequences were compared with those in the GenBank databases by using the blast and gap-blast programs (23, 24).
Representational difference analyses were performed between the chromosomal DNA of V. cholerae classical strain O395 and El Tor strain C6709 (25).
Construction of pJAS6 and pWL127.
A 248-bp internal fragment from El Tor strain N16961 of rtxC, with BglII restriction sites added at both ends, was amplified by using PCR and cloned into the BglII site of vector pGP704 (26) to obtain pJAS6. An internal fragment of rtxA was subcloned from the cosmid clone pGP65 (27) into the polylinker of pBluescriptII (Stratagene) using restriction enzymes HindIII and EcoRI to obtain pWL80. This 4-kb insert in pWL80 was then excised by SalI and EcoRI digestion and ligated into the polylinker of pGP704 (26) to generate pWL127.
These plasmids were conjugated into N16961; exconjugants were selected for ampicillin resistance, and correct integration into either rtxA or rtxC was confirmed by using PCR and Southern blot analysis.
Cytotoxicity Assay.
HEp-2 (ATCC F-13959) cells were seeded into 24-well tissue culture dishes (Costar) at 105 cells per well in RPMI medium 1640 (GIBCO/BRL) with 10% fetal calf serum and supplements (without antibiotics). Bacterial cultures grown overnight in Luria–Bertani (LB) broth at 30°C were washed twice in PBS and adjusted to about 109 cells per ml. Ten microliters of diluted bacterial culture was added to HEp-2 cells, and plates were incubated at 37°C in 5% CO2 for 1 hr. Cytotoxicity was scored by viewing live cells on an inverted microscope.
RESULTS
Identification of the RTX locus of V. cholerae.
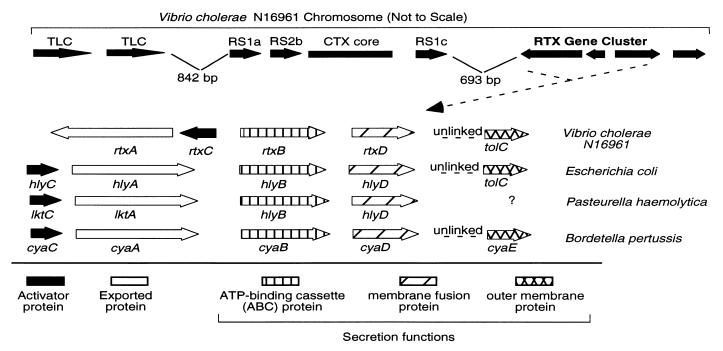
We recently identified and characterized a 4.8-kb genetic element termed pTLC (toxin linked cryptic) that is tandemly repeated in the V. cholerae genome immediately upstream of the integrated CTX prophage (20). Because the CTX element corresponds in part to the genome of a filamentous bacteriophage (28) and the TLC element encodes a gene II/X homologue found in other filamentous phages, we asked whether DNA downstream of the TLC and CTX elements encodes additional filamentous phage-related gene products. We analyzed sequence data from the Vibrio cholerae genome project (www.tigr.org) for contigs located immediately downstream of RS1c in the CTX element of the El Tor strain N16961 (Fig. 1). Contigs asm828 and asm973 span the desired region. Analysis of the ORFs within this region using the blast algorithm (23, 24) revealed a cluster of four genes that displays a high degree of similarity to genes involved in the biogenesis of RTX toxins in several Gram-negative organisms, including E. coli O157:H7 (Fig. 1) (18, 29). This cluster is located 693 nucleotides downstream of the rstB gene of the CTX element. This physical linkage of RTX to the CTX element was confirmed by using PCR with primers spanning the junction and Southern blot analysis of genomic DNA and cosmids known to contain the CTX element (data not shown).
Figure 1.
Genomic structure of the RTX element in the V. cholerae N16961 chromosome. (Upper) Schematic representation of the RTX element and its physical relationship to the nearby CTX and TLC elements in the V. cholerae El Tor N16961 genome. (Lower) Lineup of this RTX gene cluster with the RTX elements from three other Gram-negative organisms. The known or predicted functions of the ORFs within the gene clusters are indicated by different shadings.
Following convention established for known RTX toxins, we named these ORFs rtxA, rtxC, rtxB, and rtxD (Fig. 1). In the case of the E. coli hemolysin and most other RTX toxins, the genes required for toxin synthesis and secretion exist in one operon plus one additional unlinked gene. Within the operon, the order of the genes is CABD, where gene A encodes the toxin, genes B and D encode two secretion proteins, and gene C encodes the toxin activator. The operon structure of V. cholerae is unique in that an inversion of the rtxC and rtxA genes results in these genes being transcribed divergently from rtxB and rtxD (Fig. 1). In E. coli, an additional gene, tolC, is required for toxin secretion. We identified a putative tolC homologue in the V. cholerae genome as an ORF on contigasm848.
The predicted amino acid sequences of the ORFs in the V. cholerae RTX gene cluster show significant similarity to members of the RTX family. For example, blast search with RtxC sequence identified HlyC of E. coli with an E value of 2 × 10−13. The similarities for secretion gene products are even more significant: the E value between RtxB and HlyB is 2 × 10−89 and between RtxD and HlyD is 1 × 10−57. On the other hand, the E value between RtxA and HlyA is only 1 × 10−9. This lower E value is not surprising given their difference in size and activity: RtxA is more than four times the size of HlyA and does not appear to contain hemolytic activity.
A Deletion Within the RTX Gene Cluster Has Occurred in the Classical Biotype.
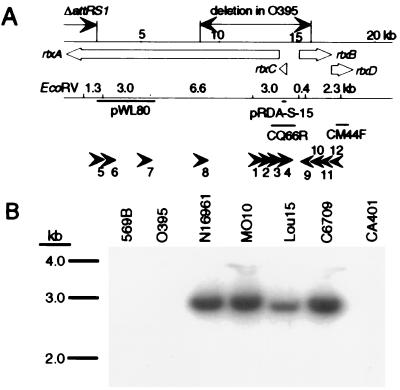
Recently Calia et al. (25) performed representational difference analysis (RDA) on two biotypes of V. cholerae to clone DNA segments that are present in the El Tor biotype but absent in the classical biotype. DNA sequence analysis of one RDA-derived DNA fragment (pRDA-S-15) revealed striking similarity to hlyC gene homologues of several RTX toxin operons (J.A.S. and S.B.C., unpublished results). Further comparison of this 393-bp fragment against sequences in the TIGR (The Institute for Genomic Research) database of partially sequenced V. cholerae El Tor N16961 genome showed that this RDA-derived segment corresponds to an ORF within contig gvc CQ66R (www.tigr.org), and that this ORF is identical to rtxC. Thus, these data suggested that rtxC is present in the El Tor strains N16961 and C6709 but is absent from the classical biotype strain O395.
We confirmed that rtxC is indeed absent from O395 by using Southern blot analysis. Based on the restriction map of the El Tor N16961 sequence, we predicted that digestion of V. cholerae genomic DNA with EcoRV generates a 3-kb fragment containing rtxC (Fig. 2). By using the RDA clone pRDA-S-15, we probed EcoRV-digested genomic DNA from seven V. cholerae strains. The high-stringency hybridization patterns indicated that three classical strains contain a deletion involving the 3-kb EcoRV fragment (Fig. 2). To map the extent of this deletion, the genomic DNA from classical and El Tor strains were probed with pWL80, which contains a 4-kb fragment in the 3′ half of rtxA, and CM44F, which contains a 1-kb fragment internal to rtxD. The results showed that the deletion removes the 5′ end of rtxA and the other end resides in rtxB (data not shown).
Figure 2.
Location of the deletion within the RTX gene cluster of V. cholerae O395. (A) The chromosomal region of V. cholerae N16961 containing the RTX element is presented schematically. The ORFs within the region are represented by open arrows and labeled with the names of the genes they are predicted to encode. A line above the arrows provides a scale with the length indicated in kb. The extent of the deletion within the O395 RTX region is marked (↔). The ΔattRS1 deletion in the candidate vaccine strains has also been indicated. A line below the ORF map shows the position of EcoRV sites within the region, with the predicted fragment sizes produced by an EcoRV digest indicated in kb. Beneath this line are solid bars that represent the different DNA fragments cloned and used in this study and a set of small arrowheads that correspond to sites where different primers anneal. (B) Autoradiograph showing the Southern blot analysis of V. cholerae genomic DNA digested with EcoRV and hybridized with pRDA-S-15 probe. 569B, O395, and CA401 are of O1 serotype and classical biotype; N16961, Lou15, and C6709 are O1 serotype and El Tor biotype; and MO10 is an O139 serotype strain.
To refine the analysis, we amplified the deletion junctions in the classical strain O395 by PCR with a set of nested primers (Fig. 2). Sequencing the smallest amplified fragment revealed a 7,869-bp deletion from nucleotide 5635 of rtxA to nucleotide 954 of rtxB in the O395 genome (Fig. 2). This result confirmed that the deletion removes the 5′ end of rtxA, all of rtxC, the 5′ end of rtxB, and any regulatory element that lies between these divergently transcribed genes.
PCR analysis of the deletion junctions in several other classical and El Tor strains demonstrated a strict biotype segregation: sequences are absent in classical strains 569B, CA401, and NIH41, and present in El Tor strains P27459, E7946, and C6709 (data not shown). The gene cluster also is intact in the O139 strain MO10, consistent with the presumed derivation of this serotype from the serotype O1 El Tor strains (30). Furthermore, the RTX gene cluster is intact in several environmental isolates of V. cholerae from the Gulf coast, including nonpathogenic strains that lack the CTX element (data not shown), suggesting that presence of the RTX gene cluster predates acquisition of the neighboring CTX element.
Functional Characterization of the RTX ORFs.
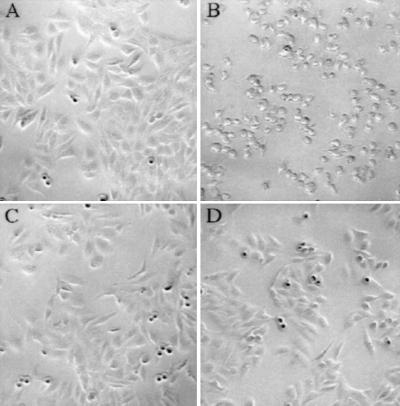
RTX toxins produced by other Gram-negative bacteria are typically soluble, secreted proteins that display cytotoxic or hemolytic activity (18, 29). Attempts to demonstrate cytotoxic activity in filtered supernatant fluids of V. cholerae were unsuccessful. However, washed bacterial cells of some strains display cytotoxic activity against a variety of tissue culture cells (data not shown). For example, within 1 hr of exposure to wild-type bacteria of the El Tor biotype, the majority of cultured HEp-2 cells become spherical and detach (Fig. 3), whereas HEp-2 cells can withstand 3 hr of exposure to the classical biotype (data not shown).
Figure 3.
The cytotoxic effect of RTX+ and RTX− strains on HEp-2 cells in culture. Subconfluent HEp-2 monolayers were exposed for 1 hr to PBS-washed V. cholerae cells at a multiplicity of infection (MOI) of 100. The V. cholerae strains added were mock (PBS) (A), N16961 (B), WLV12 (C), and JAS6 (D).
An El Tor strain defective in producing hemagglutinin/protease (HA/P, encoded by the hap gene) (31) expresses the same cytotoxic activity, demonstrating that HA/P is not responsible for the cytotoxicity (Table 2). However, certain candidate El Tor vaccine strains developed in our laboratory do lack this activity. These strains contain a deletion between the two HindIII sites which bracket the CTX element (ΔattRS1), removing the last 1,907-bp of rtxA (Fig. 4). Other El Tor strains bearing deletion of only the core CTX element (Δcore) retain their cytotoxic activity, demonstrating that the activity is not caused by cholera toxin (CT), zonula occludens toxin (Zot), or accessary cholera enterotoxin (Ace). Because both classical strains and ΔattRS1 El Tor vaccine strains contain deletions in the RTX gene cluster, we surmised that cytotoxicity in other V. cholerae El Tor and O139 strains is caused by the presence of an intact RTX gene cluster.
Figure 4.
The potential structural domains and motifs in V. cholerae RtxA and sequence comparison to other RTX toxins. (A) The predicted RtxA polypeptide is represented schematically by an open box with its length in amino acid residues indicated on the line above. The positions of potential ATP/GTP-binding domains and the two RGD motifs are indicated. Larger putative structual domains are identified by solid bars. (B) Sequence alignments of repeats from the hydrophobic region (amino acids 781-1332) and repeats from the GD-rich region (amino acids 4165–4362) of V. cholerae RtxA.
To test this hypothesis, we disrupted the rtxA gene in a series of strains by integrating pWL127, which contains an internal HindIII-EcoRI fragment of rtxA gene, into the V. cholerae genome via homologous recombination (data not shown). The parental strains included classical and El Tor clinical strains isolated from different continents over several decades, and an O139 isolate from southern India in 1993 (Table 1). When assayed on HEp-2 cells, only strains with an intact rtxA elicited cytotoxicity. Assays conducted with El Tor strains disrupted in either rtxA or rtxC gene showed no cytotoxicity, consistent with the role of RtxA in cytotoxicity and the presumed role of RtxC in activating RtxA (Fig. 3).
Structural Features of RtxA.
The RTX toxins as a group have certain common domain structures: an N-terminal hydrophobic domain required for pore formation; central prototoxin activation sites; C-terminal GD-rich calcium-binding repeats involved in target-cell binding; and a C-terminal signal for RtxB/RtxD-dependent secretion.
Analysis of the deduced amino acid sequence of RtxA shows some similar as well as distinguishing features (Fig. 4). RtxA is 4,546 aa in size, compared with about 1,000 residues for the E. coli HlyA. A pore-forming domain is notably absent in the N terminus of RtxA, although a more internal hydrophobic region (amino acids 600-1200) may serve the pore-forming function. This hydrophobic region overlaps with a region rich in repeats (amino acids 700-1350); however, these repeats are remarkably different in sequence from the nine-residue GD-rich repeats commonly found in RTX toxins (Fig. 4). These repeats consist of 19 amino acids with the consensus GXAN(I/V)XT(K/H)VGDGXTVAVMX. This motif is repeated consecutively 29 times in RtxA and shares no sequence similarity to any other motif or protein domain in the current database. Furthermore, the hydrophobic regions of other RTX toxins do not contain many repeats. The actual GD-rich repeats of RtxA are located toward the C terminus as in E. coli HlyA. In fact, this C-terminal repeat region of RtxA shows the greatest sequence similarity to other RTX toxins (Fig. 4). However, instead of the usual 9-residue repeats, these 9 residues are preceded by an additional 9 residues, resulting in an 18-residue consensus motif X(V/I)XXGXXNX(V/I)XXGDGXDX.
Within the enormous length of RtxA, about a dozen sequences conform to the consensus for toxin activation by acylation (32). However, whether or how many of these sites are substrates for acylation is unknown. Finally, the C terminus of the protein may contain a secretion signal. Although the secretion signals of RTX toxins do not follow a strong consensus, disruption of an “aspartate box” motif, rich in aspartic acid and serine, at the C terminus of other RTX toxins can severely affect secretion (33, 34). In V. cholerae RtxA, 7 aspartate and 9 serine residues are present within the last 50 aa.
Although large regions of the protein do not share sequence similarity to proteins in the database, certain motifs are present. Two RGD sequences appear at amino acids 1810 and 4214 of RtxA (Fig. 4). This short peptide sequence, found in fibronectin and other adhesive proteins, facilitates binding to the integrin family of cell surface receptors (35). Whether RGD sequences have any role in activity of RtxA is unknown. Toward the N terminus, at amino acids 524 and 1080, are two putative eight-amino acid ATP/GTP-binding domains (Fig. 4), which is interesting because at least one RTX toxin, the CyaA toxin of Bordetella pertussis, is known to be an adenylate cyclase. However, RtxA of V. cholerae does not share extensive sequence similarity with the N-terminal cyclase domain of CyaA.
DISCUSSION
We identified a toxin in V. cholerae that belongs to the RTX family of hemolysins/leukotoxins by using a combination of genomic sequence analysis and representational difference analysis. This RTX gene cluster encodes the presumptive cytotoxin RtxA, an acyltransferase RtxC, and an associated ABC transporter system RtxB/D. It is physically linked to the CTX element on the V. cholerae genome, although its activity is independent of the CTX element. Strains of the classical biotype contain a deletion within this gene cluster, whereas in all other strains we examined the genes are intact. The presence of an intact RTX gene cluster is correlated with the expression of a cell-associated, cytotoxic activity that causes mammalian epithelial HEp-2 cells to detach and round-up.
Several groups have identified cytotoxic activities expressed by O1 El Tor and non-O1 strains of V. cholerae that are independent of the CTX element (36–38). However, the characteristics of these toxins differ biochemically from the RTX toxin reported here. For example, one group identified a V. cholerae cytotoxic activity that is cell-associated and that causes the rounding of tissue culture cells similar to the activity found for RtxA (39). However, the denatured form of this toxin shows a molecular mass of 35 kDa, far below the 500-kDa predicted size of RtxA. Although the gene encoding this small toxin has not been identified, it seems unlikely that the 4,546-aa primary translation product of RtxA would be processed to an active fragment of only 35 kDa.
We do not know how RtxA exerts its cytotoxicity. It may introduce pores into the membrane of target cells by inserting its hydrophobic domain like other cytotoxins. Alternatively, this large polypeptide may have a novel domain that contains the cytotoxic activity. Fernandez-Prada et al. (40) recently reported that α-hemolysin of E. coli can cause death of macrophages by two different modes depending on the cell line: by damaging the plasma membrane integrity without changing the nuclear morphology or by inducing apoptosis. In another report, Lally et al. (41) identified β2 integrin as the cell-surface receptor for the RTX toxins from Actinobacillus actinomycetemcomitans and E. coli. Given the presence of two RGD motifs within RtxA, it may also interact with the target cells by binding to host integrins.
The RTX toxin of V. cholerae is encoded within a gene cluster that is physically linked to the cholera toxin prophage (28). Because genes of common pathogenic function are often genetically linked, we propose that the V. cholerae RTX toxin, like cholera toxin, may play a role in the gastrointestinal virulence properties of V. cholerae. Classical strains of V. cholerae are defective in production of cytotoxic activity associated with the RTX gene cluster because they carry a deletion of DNA sequences that overlap the rtxA, rtxC, and rtxB genes. Classical strains also carry defective CTX prophage sequences and other defective prophages (42). The accumulation of defective gene sequences such as those in the RTX region may reflect the evolutionary age of the classical biotype. It is generally thought that classical strains are more ancient pathogens than pandemic El Tor O1 and O139 strains, which emerged in 1961 and 1992, respectively. The fact that El Tor and O139 strains have all but completely displaced the older classical biotype as agents of cholera (1–6) further suggests that the intact RTX gene cluster might have provided a selective advantage for these newer strains during the process of emergence and displacement.
The RTX toxin may also contribute to the reactogenicity of some V. cholerae vaccine strains in human subjects. Strains such as JBK70, CVD110, CVD111, and CVD112 display significant reactogenicity in volunteers despite the fact that they are deleted in part or all of the core element (Δcore) including cholera toxin and other putative toxins encoded by the CTX prophage (13, 14, 43). However, these simple deletions leave the RTX gene cluster intact. On the other hand, certain El Tor and O139 vaccine strains carrying so called “attRS1 deletions” (10, 12, 16) have lost 14% of the rtxA coding sequence, and we showed that these ΔattRS1 deletion strains do not express RtxA activity in vitro (Table 1). This loss of RtxA activity may partially explain why these ΔattRS1 strains show reduced reactogenicity in human subjects compared with other El Tor and O139 vaccine strains that express functional RtxA. Furthermore, two older vaccine derivatives of classical biotype strains, CVD103 and O395-N1 (44, 45), which are predicted to be deficient in production of active RtxA, also display considerably less reactogenicity in volunteers than the RTX-positive El Tor O1 and O139 derivatives noted above (13, 14, 43–45).
In addition, the V. cholerae RtxA may be responsible for inducing a local intestinal inflammatory response in volunteers immunized with RTX-positive vaccine strains. Recently, Silva et al. (46) observed that the diarrheal stool of volunteers colonized with reactogenic El Tor vaccine strain CVD110 contains copious amounts of lactoferrin, a reliable marker for inflammatory diarrhea (47). A role for RtxA in this process is suggested by the observation that several RTX toxins induce the production of proinflammatory substances such as IL-1, TNF, nitric oxide, and eicosanoids (48). To prove that the RtxA of V. cholerae plays a role in the residual virulence of CTX-negative vaccine prototypes, isogenic constructs of reactogenic strains will need to be tested that are deleted in the entire rtxA coding sequence.
Acknowledgments
We thank members of the Mekalanos and Calderwood labs for invaluable help and constructive input on this work; we also thank Peter Rahaim and members of the Microbiology DNA sequencing Core Facility. This work was supported by the National Institutes of Health Grants AI26289 (J.J.M.) and AI40725 (S.B.C). W.L. is the recipient of a Predoctoral Fellowship of the Howard Hughes Medical Institute. K.J.F. is funded by National Research Service Award Postdoctoral Training Grant AI07410–06.
ABBREVIATIONS
- RTX
repeat in toxin
- CTX
cholera toxin genetic element
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF119150).
References
- 1.Tauxe R, Seminario L, Tapia R, Libel M. In: Vibrio cholerae and Cholera: Molecular to Global Perspectives. Wachsmuth I K, Blake P A, Olsvik Ø, editors. Washington, DC: Am. Soc. Microbiol.; 1994. pp. 321–344. [Google Scholar]
- 2.Swerdlow D L, Isaäcson M. In: Vibrio cholerae and Cholera: Molecular to Global Perspectives. Wachsmuth I K, Blake P A, Olsvik Ø, editors. Washington, DC: Am. Soc. Microbiol.; 1994. pp. 297–308. [Google Scholar]
- 3.Glass R I, Black R E. In: Current Topics in Infectious Disease: Cholera. Barua D, Greenough W B, editors. New York: Plenum; 1992. pp. 129–154. [Google Scholar]
- 4.Wachsmuth K, Blake P A, Olsvik Ø. Vibrio cholerae and Cholera: Molecular to Global Perspectives. Washington, DC: Am. Soc. Microbiol.; 1994. [Google Scholar]
- 5.Albert M J, Siddique A K, Islam M S, Faruque A S G, Ansaruzzaman M, Faruque S M, Sack R B. Lancet. 1993;341:704. doi: 10.1016/0140-6736(93)90481-u. [DOI] [PubMed] [Google Scholar]
- 6.Ramamurthy T, Garg S, Sharma R, Bhattacharya S K, Nair G B, Shimada T, Takeda T, Karasawa T, Kurazano H, Pal A, Takeda Y. Lancet. 1993;341:703–704. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- 7.Johnson J A, Salles C A, Panigrahi P, Albert M J, Wright A C, Johnson R J, Morris J G. Infect Immun. 1994;62:2108–2110. doi: 10.1128/iai.62.5.2108-2110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mekalanos J J, Swartz D J, Pearson G D, Harford N, Groyne F, deWilde M. Nature (London) 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 9.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coster T S, Killeen K P, Waldor M K, Beattie D, Spriggs D, Kenner J R, Trofa A, Sadoff J, Mekalanos J J, Taylor D N. Lancet. 1995;345:949–952. doi: 10.1016/s0140-6736(95)90698-3. [DOI] [PubMed] [Google Scholar]
- 11.Levine M M, Kaper J B, Herrington D, Losonsky G, Morris J G, Clements M, Black R E, Tall B, Hall R. Infect Immun. 1988;56:161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenner J, Coster T, Trofa A, Taylor D, Barrera-Oro M, Hyman T, Adams J, Beattie D, Killeen K, Mekalanos J J, Sadoff J C. J Infect Dis. 1995;172:1126–1129. doi: 10.1093/infdis/172.4.1126. [DOI] [PubMed] [Google Scholar]
- 13.Tacket C, Losonsky G, Nataro J, Comstock L, Michalski J, Edelman R, Kaper J, Levine M. J Infect Dis. 1995;172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 14.Tacket C O, Kotloff K L, Losonsky G, Nataro J P, Michalski J, Kaper J B, Edelman R, Levine M M. Am J Trop Med Hyg. 1997;56:533–537. doi: 10.4269/ajtmh.1997.56.533. [DOI] [PubMed] [Google Scholar]
- 15.Tacket C O, Losonsky G, Nataro J P, Cryz S J, Edelman R, Fasano A, Michalski J, Kaper J B, Levine M M. J Infect Dis. 1993;168:1536–1540. doi: 10.1093/infdis/168.6.1536. [DOI] [PubMed] [Google Scholar]
- 16.Taylor D N, Killeen K P, Hack D C, Kenner J R, Coster T S, Beattie D T, Ezzell J, Hyman T, Trofa A, Sjogren M H, et al. J Infect Dis. 1994;170:1518–1523. doi: 10.1093/infdis/170.6.1518. [DOI] [PubMed] [Google Scholar]
- 17.Fox J L. ASM News. 1998;64:439–440. [Google Scholar]
- 18.Braun V, Schonherr R, Hobbie S. Trends Microbiol. 1993;1:211–216. doi: 10.1016/0966-842x(93)90134-d. [DOI] [PubMed] [Google Scholar]
- 19.Welch R A, Bauer M E, Kent A D, Leeds J A, Moayeri M, Regassa L B, Swenson D L. Infect Agents Dis. 1995;4:254–272. [PubMed] [Google Scholar]
- 20.Rubin E J, Lin W, Mekalanos J J, Waldor M K. Mol Microbiol. 1998;28:1247–1254. doi: 10.1046/j.1365-2958.1998.00889.x. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 22.Ausubel F M, Brent R, Kingston R E, Moore D E, Seidman J G, Smith J A, Struhl K. In: Current Protocols in Molecular Biology. Chanda V B, editor. New York: Wiley; 1995. [Google Scholar]
- 23.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 24.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calia K E, Waldor M K, Calderwood S B. Infect Immun. 1998;66:849–852. doi: 10.1128/iai.66.2.849-852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller V L, Mekalanos J J. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson G D N. Ph.D. thesis. Cambridge, MA: Harvard Univ. Press; 1989. [Google Scholar]
- 28.Waldor M K, Mekalanos J J. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 29.Bauer M E, Welch R A. Infect Immun. 1996;64:167–175. doi: 10.1128/iai.64.1.167-175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waldor M K, Mekalanos J J. J Infect Dis. 1994;170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]
- 31.Hase C C, Finkelstein R A. J Bacteriol. 1991;173:3311–3317. doi: 10.1128/jb.173.11.3311-3317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellett S, Welch R A. Infect Immun. 1996;64:3081–3087. doi: 10.1128/iai.64.8.3081-3087.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenny B, Taylor S, Holland I B. Mol Microbiol. 1992;6:1477–1489. doi: 10.1111/j.1365-2958.1992.tb00868.x. [DOI] [PubMed] [Google Scholar]
- 34.Gray L, Baker K, Kenny B, Mackman N, Haigh R, Holland I B. J Cell Sci Suppl. 1989;11:45–57. doi: 10.1242/jcs.1989.supplement_11.4. [DOI] [PubMed] [Google Scholar]
- 35.Ruoslahti E. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 36.Honda T, Finkelstein R A. Infect Immun. 1979;26:1020–1027. doi: 10.1128/iai.26.3.1020-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichinose Y, Yamamoto K, Nakasone N, Tanabe M J, Takeda T, Miwatani T, Iwanaga M. Infect Immun. 1987;55:1090–1093. doi: 10.1128/iai.55.5.1090-1093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto K, Ichinose Y, Nakasone N, Tanabe M, Nagahama M, Sakurai J, Iwanaga M. Infect Immun. 1986;51:927–931. doi: 10.1128/iai.51.3.927-931.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saha P K, Koley H, Nair G B. Infect Immun. 1996;64:3101–3108. doi: 10.1128/iai.64.8.3101-3108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez-Prada C, Tall B D, Elliott S E, Hoover D L, Nataro J P, Venkatesan M M. Infect Immun. 1998;66:3918–3924. doi: 10.1128/iai.66.8.3918-3924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lally E T, Kieba I R, Sato A, Green C L, Rosenbloom J, Korostoff J, Wang J F, Shenker B J, Ortlepp S, Robinson M K, Billings P C. J Biol Chem. 1997;272:30463–9. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 42.Gerdes J C, Romig W R. Infect Immun. 1975;11:445–452. doi: 10.1128/iai.11.3.445-452.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tacket C O, Losonsky G, Nataro J P, Cryz S J, Edelman R, Fasano A, Michalski J, Kaper J B, Levine M M. J Infect Dis. 1993;168:1536–1540. doi: 10.1093/infdis/168.6.1536. [DOI] [PubMed] [Google Scholar]
- 44.Herrington D A, Hall R H, Losonsky G, Mekalanos J J, Taylor R K, Levine M M. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine M M, Kaper J B, Herrington D, Ketley J, Losonsky G, Tacket C O, Tall B, Cryz R. Lancet. 1988;ii:467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- 46.Silva T M, Schleupner M A, Tacket C O, Steiner T S, Kaper J B, Edelman R, Guerrant R. Infect Immun. 1996;64:2362–2364. doi: 10.1128/iai.64.6.2362-2364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scerpella E G, Okhuysen P C, Mathewson J J, Guerrant R L, Latimer E, Lyerly D, Ericsson C D. J Travel Med. 1994;1:4–7. doi: 10.1111/j.1708-8305.1994.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 48.Czuprynski C J, Welch R A. Trends Microbiol. 1995;3:480–483. doi: 10.1016/s0966-842x(00)89016-2. [DOI] [PubMed] [Google Scholar]