Abstract
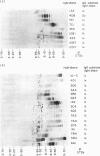
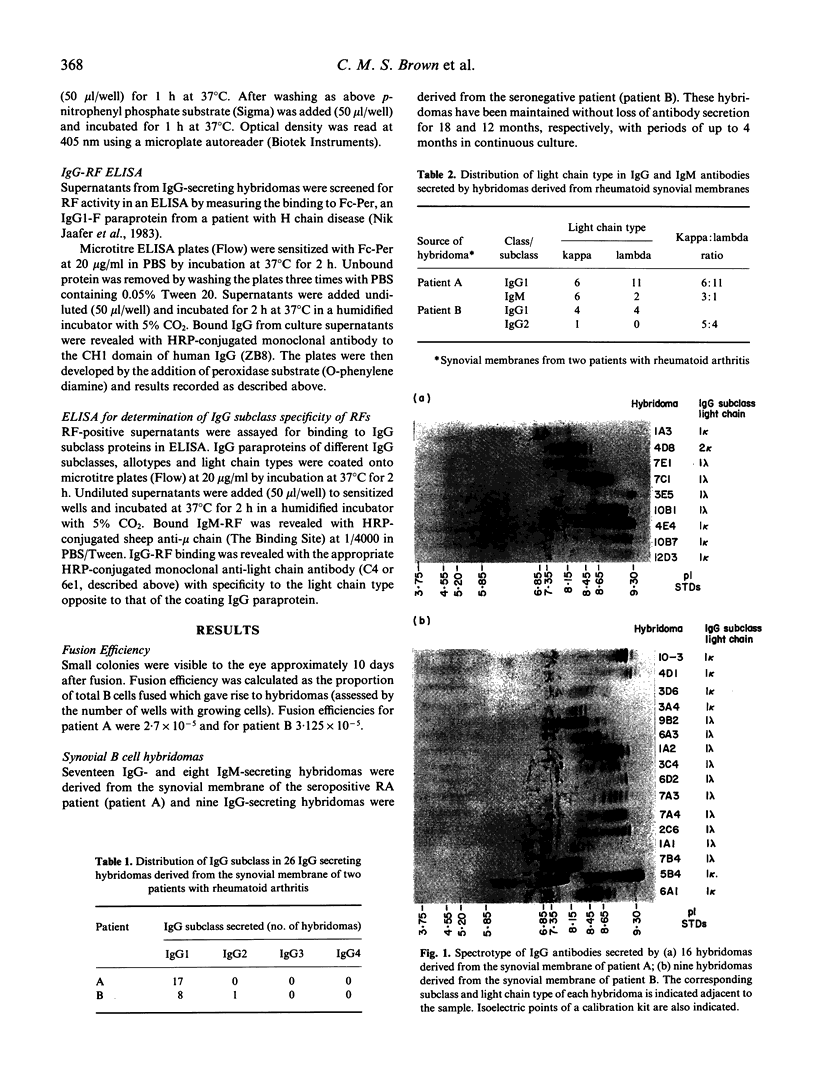
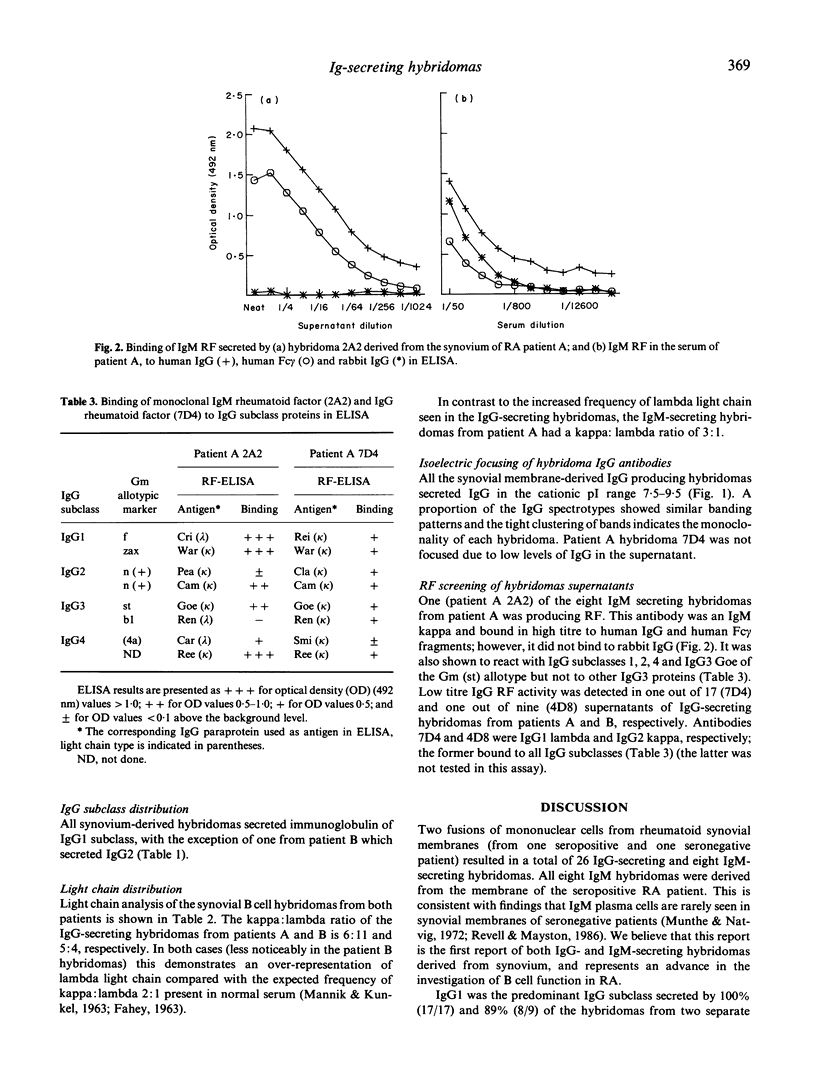
Twenty-six IgG-secreting and eight IgM-secreting hybridomas were derived from the synovia of two patients with rheumatoid arthritis (RA). Hybridomas were obtained by fusing a heteromyeloma cell line, SPAZ-4 with synovial mononuclear cells that were not deliberately stimulated in vitro. Over 96% of the IgG-secreting hybridomas produced antibodies which belonged to the IgG1 subclass and showed lambda light chain predominance; the latter was not seen in IgM antibodies, where kappa light chains dominated by 3:1. All IgG antibodies were cationic. Synovial B cells were not exposed to extrinsic stimuli prior to fusion, therefore these results reflect the state of B cell activation and differentiation in vivo. Our results indicate that IgG-secreting B cells in the RA joint are under a selective influence which is, as yet, unidentified. One out of eight IgM-secreting and two out of 26 IgG-secreting hybridomas produced rheumatoid factors (RF). The IgM-RF specificity for IgG heavy chain subclasses was determined and showed that the monoclonal bound to IgG1, IgG2 and IgG4 but not IgG3 with exception of IgG3 Goe of the G3m (st) allotype, a profile typical of specificity for the Ga epitope. This monoclonal also distinguished a determinant in the Fc region of human IgG which was not present in rabbit IgG. The overall frequency of RF-secreting hybridomas we observed indicates that B cells committed to RF production in the synovium of a seropositive and a seronegative RA patient is below 10%.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. C., Kunkel H. G. Hidden rheumatoid factors with specificity for native gamma globulins. Arthritis Rheum. 1966 Dec;9(6):758–768. doi: 10.1002/art.1780090603. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- DIAGNOSTIC criteria for rheumatoid arthritis: 1958 revision by a committee of the American Rheumatism Association. Ann Rheum Dis. 1959 Mar;18(1):49–53. [PMC free article] [PubMed] [Google Scholar]
- De Blas A. L., Cherwinski H. M. Detection of antigens on nitrocellulose paper immunoblots with monoclonal antibodies. Anal Biochem. 1983 Aug;133(1):214–219. doi: 10.1016/0003-2697(83)90245-2. [DOI] [PubMed] [Google Scholar]
- Ebling F., Hahn B. H. Restricted subpopulations of DNA antibodies in kidneys of mice with systemic lupus. Comparison of antibodies in serum and renal eluates. Arthritis Rheum. 1980 Apr;23(4):392–403. doi: 10.1002/art.1780230402. [DOI] [PubMed] [Google Scholar]
- Epstein W. V., Tan M. Increase of L-chain proteins in the sera of patients with systemic lupus erythematosus and the synovial fluids of patients with peripheral rheumatoid arthritis. Arthritis Rheum. 1966 Oct;9(5):713–719. doi: 10.1002/art.1780090508. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L. TWO TYPES OF 6.6 S GAMMA-GLOBULINS, BETA-2A-GLOBULINS AND 18 S GAMMA 1-MACROGLOBULINS IN NORMAL SERUM AND GAMMA-MICROGLOBULINS IN NORMAL URINE. J Immunol. 1963 Oct;91:438–447. [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimizu M., Maini R., Feldmann M. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988 Nov;18(11):1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Hoffman W. L., Douglass R. R., Smiley J. D. Synthesis of electrophoretically restricted IgG by cultured rheumatoid synovium. Arthritis Rheum. 1984 Sep;27(9):976–984. doi: 10.1002/art.1780270903. [DOI] [PubMed] [Google Scholar]
- Hoffman W. L., Goldberg M. S., Smiley J. D. Immunoglobulin G3 subclass production by rheumatoid synovial tissue cultures. J Clin Invest. 1982 Jan;69(1):136–144. doi: 10.1172/JCI110424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunneyball I. M., Stanworth D. R. The effects of chemical modification on the antigenicity of human and rabbit immunoglobulin G. Immunology. 1976 Jun;30(6):881–894. [PMC free article] [PubMed] [Google Scholar]
- Jaafar M. I., Lowe J. A., Ling N. R., Jefferis R. Immunogenic and antigenic epitopes of immunoglobulins--V. Reactivity of a panel of monoclonal antibodies with sub-fragments of human Fc gamma and abnormal paraproteins having deletions. Mol Immunol. 1983 Jun;20(6):679–686. doi: 10.1016/0161-5890(83)90012-3. [DOI] [PubMed] [Google Scholar]
- Jefferis R., Nik Jaafar M. I., Steinitz M. Immunogenic and antigenic epitopes of immunoglobulins. VIII. A human monoclonal rheumatoid factor having specificity for a discontinuous epitope determined by histidine/arginine interchange as residue 435 of immunoglobulin G. Immunol Lett. 1984;7(4):191–194. doi: 10.1016/0165-2478(84)90042-7. [DOI] [PubMed] [Google Scholar]
- Johnson P. M., Faulk W. P. Rheumatoid factor: its nature, specificity, and production in rheumatoid arthritis. Clin Immunol Immunopathol. 1976 Nov;6(3):414–430. doi: 10.1016/0090-1229(76)90094-5. [DOI] [PubMed] [Google Scholar]
- Lindström F. D. Kappa:lambda light chain ratio in IgG eluted from rheumatoid arthritis synovium. Clin Exp Immunol. 1970 Jul;7(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Ling N. R., Bishop S., Jefferis Use of antibody-coated red cells for the sensitive detection of antigen and in rosette tests for cells bearing surface immunoglobulins. J Immunol Methods. 1977;15(3):279–289. doi: 10.1016/0022-1759(77)90065-5. [DOI] [PubMed] [Google Scholar]
- Mayumi M., Kuritani T., Kubagawa H., Cooper M. D. IgG subclass expression by human B lymphocytes and plasma cells: B lymphocytes precommitted to IgG subclass can be preferentially induced by polyclonal mitogens with T cell help. J Immunol. 1983 Feb;130(2):671–677. [PubMed] [Google Scholar]
- Mellbye O. J., Vartdal F., Dobloug J. H. Subclasses of IgG produced in the rheumatoid synovium. Rheumatol Int. 1984;4 (Suppl):49–52. doi: 10.1007/BF00541279. [DOI] [PubMed] [Google Scholar]
- Mongini P. K., Stein K. E., Paul W. E. T cell regulation of IgG subclass antibody production in response to T-independent antigens. J Exp Med. 1981 Jan 1;153(1):1–12. doi: 10.1084/jem.153.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe E., Natvig J. B. Immunglobulin classes, subclasses and complexes of IgG rheumatoid factor in rheumatoid plasma cells. Clin Exp Immunol. 1972 Sep;12(1):55–70. [PMC free article] [PubMed] [Google Scholar]
- Natvig J. B., Munthe E. Self-associating IgG rheumatoid factor represents a major response of plasma cells in rheumatoid inflammatory tissue. Ann N Y Acad Sci. 1975 Jun 13;256:88–95. doi: 10.1111/j.1749-6632.1975.tb36038.x. [DOI] [PubMed] [Google Scholar]
- Ostberg L., Pursch E. Human X (mouse X human) hybridomas stably producing human antibodies. Hybridoma. 1983;2(4):361–367. doi: 10.1089/hyb.1983.2.361. [DOI] [PubMed] [Google Scholar]
- Plater-Zyberk C., Clarke M. F., Lam K., Mumford P. A., Room G. R., Maini R. N. In vitro immunoglobulin synthesis by lymphocytes from patients with rheumatoid arthritis. I. Effect of monocyte depletion and demonstration of an increased proportion of lymphocytes forming rosettes with mouse erythrocytes. Clin Exp Immunol. 1983 Jun;52(3):505–511. [PMC free article] [PubMed] [Google Scholar]
- Randen I., Thompson K. M., Natvig J. B., Førre O., Waalen K. Human monoclonal rheumatoid factors derived from the polyclonal repertoire of rheumatoid synovial tissue: production and characterization. Clin Exp Immunol. 1989 Oct;78(1):13–18. [PMC free article] [PubMed] [Google Scholar]
- Revell P. A., Mayston V. J. Immunoglobulin classes in plasma cells of the synovial membrane in chronic inflammatory joint disease. Ann Rheum Dis. 1986 May;45(5):405–408. doi: 10.1136/ard.45.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesen W. F., Skvaril F., Braun D. G. Natural infection of man with group A streptococci. Levels; restriction in class, subclass, and type; and clonal appearance of polysaccharide-group-specific antibodies. Scand J Immunol. 1976;5(4):383–390. doi: 10.1111/j.1365-3083.1976.tb00292.x. [DOI] [PubMed] [Google Scholar]
- Skvaril F. IgG subclasses in viral infections. Monogr Allergy. 1986;19:134–143. [PubMed] [Google Scholar]
- Slaughter L., Carson D. A., Jensen F. C., Holbrook T. L., Vaughan J. H. In vitro effects of Epstein-Barr virus on peripheral blood mononuclear cells from patients with rheumatoid arthritis and normal subjects. J Exp Med. 1978 Nov 1;148(5):1429–1434. doi: 10.1084/jem.148.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski A. J., Zvaifler N. J. In vivo synthesis of IgG by rheumatoid synovium. J Lab Clin Med. 1970 Aug;76(2):304–310. [PubMed] [Google Scholar]
- Smiley J. D., Sachs C., Ziff M. In vitro synthesis of immunoglobulin by rheumatoid synovial membrane. J Clin Invest. 1968 Mar;47(3):624–632. doi: 10.1172/JCI105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist V. A., Linde A., Wahren B. Virus-specific immunoglobulin G subclasses in herpes simplex and varicella-zoster virus infections. J Clin Microbiol. 1984 Jul;20(1):94–98. doi: 10.1128/jcm.20.1.94-98.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale J. M. Abnormalities in isotype expression in CBA/N mice due to stimulatory environment rather than a B cell defect. J Immunol. 1983 Jan;130(1):72–77. [PubMed] [Google Scholar]
- Vitetta E. S., Ohara J., Myers C. D., Layton J. E., Krammer P. H., Paul W. E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med. 1985 Nov 1;162(5):1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernick R. M., Lipsky P. E., Marban-Arcos E., Maliakkal J. J., Edelbaum D., Ziff M. IgG and IgM rheumatoid factor synthesis in rheumatoid synovial membrane cell cultures. Arthritis Rheum. 1985 Jul;28(7):742–752. doi: 10.1002/art.1780280704. [DOI] [PubMed] [Google Scholar]
- Williams D. G., Brennan F. M., Stocks M. R., Maini R. N. Individual variation of anti-Sm autoantibodies in the MRL/MP-lpr/lpr mouse: age-related increase in diversity. Clin Exp Immunol. 1986 Sep;65(3):506–512. [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Yoshida M., Izui S., Lambert P. H. Distinct clonotypes of anti-DNA antibodies in mice with lupus nephritis. J Clin Invest. 1985 Aug;76(2):685–694. doi: 10.1172/JCI112022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatarain-Rios E., Mannik M. Charge-charge interactions between articular cartilage and cationic antibodies, antigens, and immune complexes. Arthritis Rheum. 1987 Nov;30(11):1265–1273. doi: 10.1002/art.1780301109. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]