Abstract
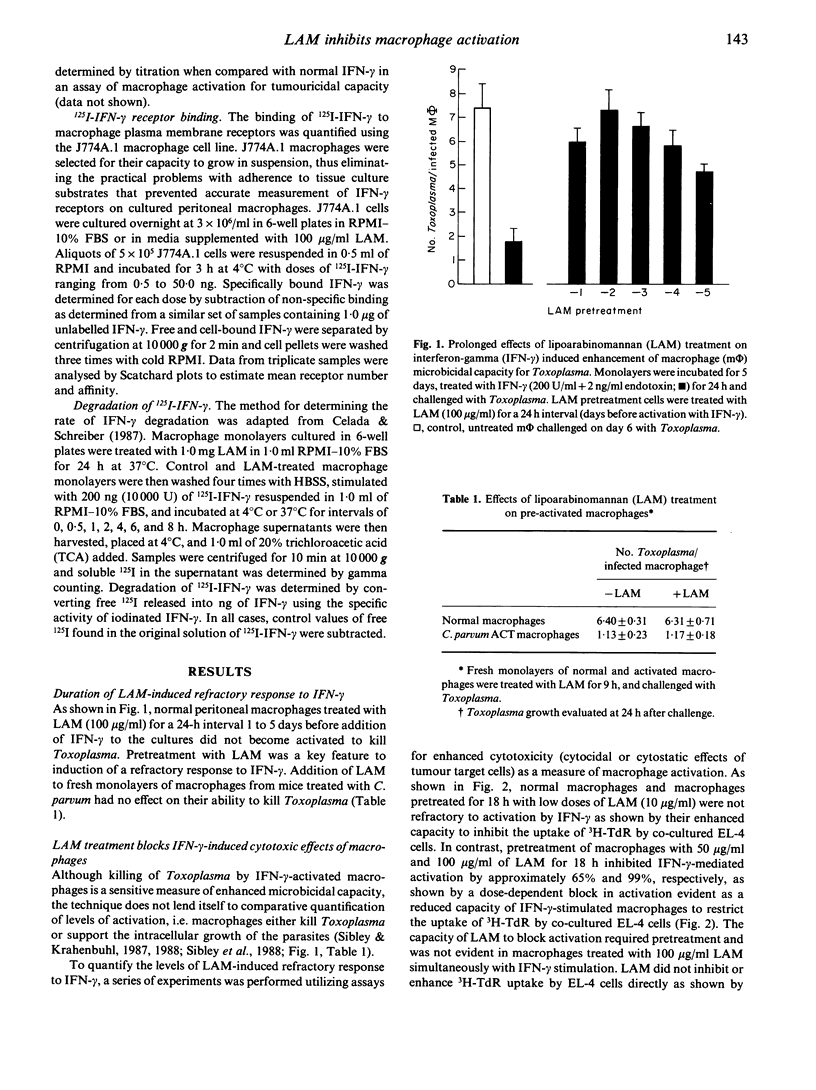
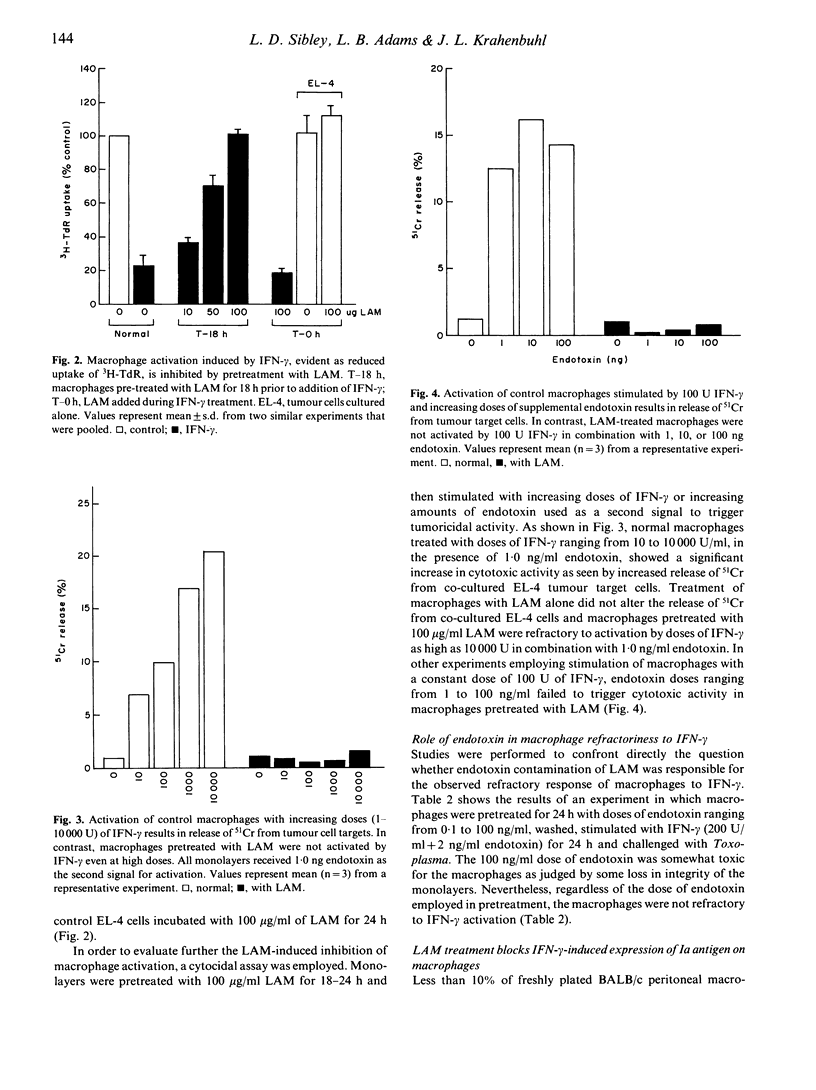
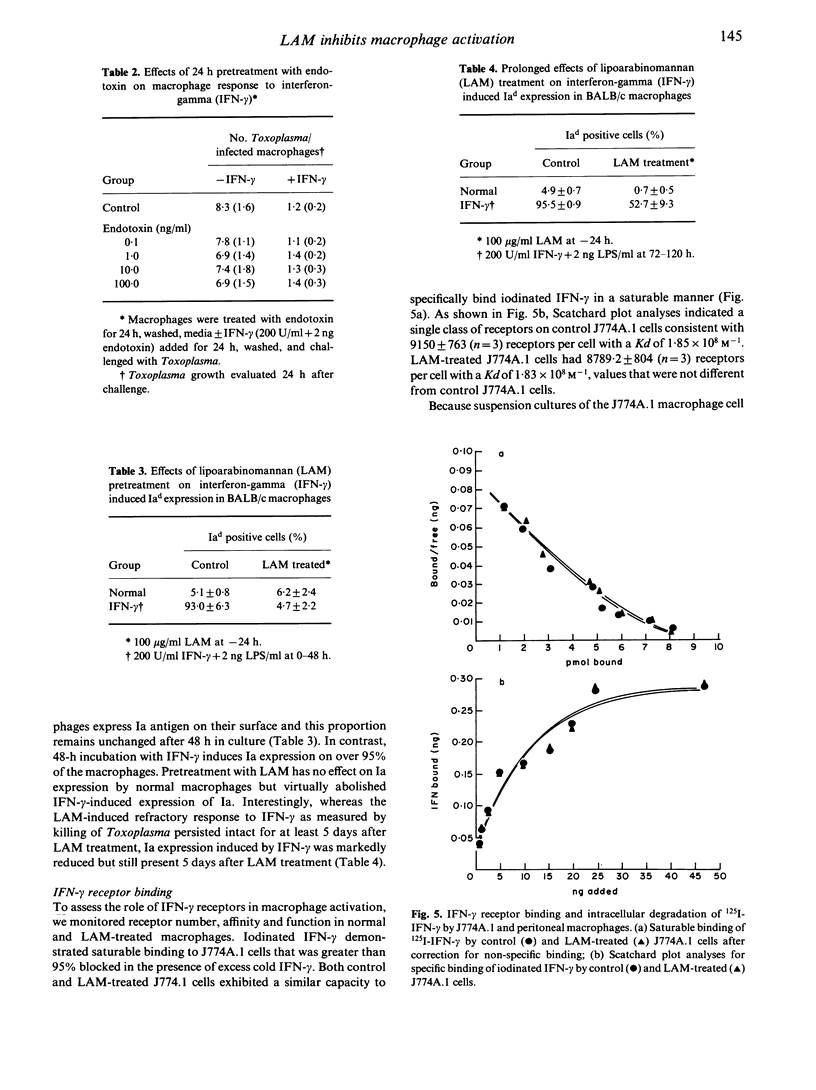
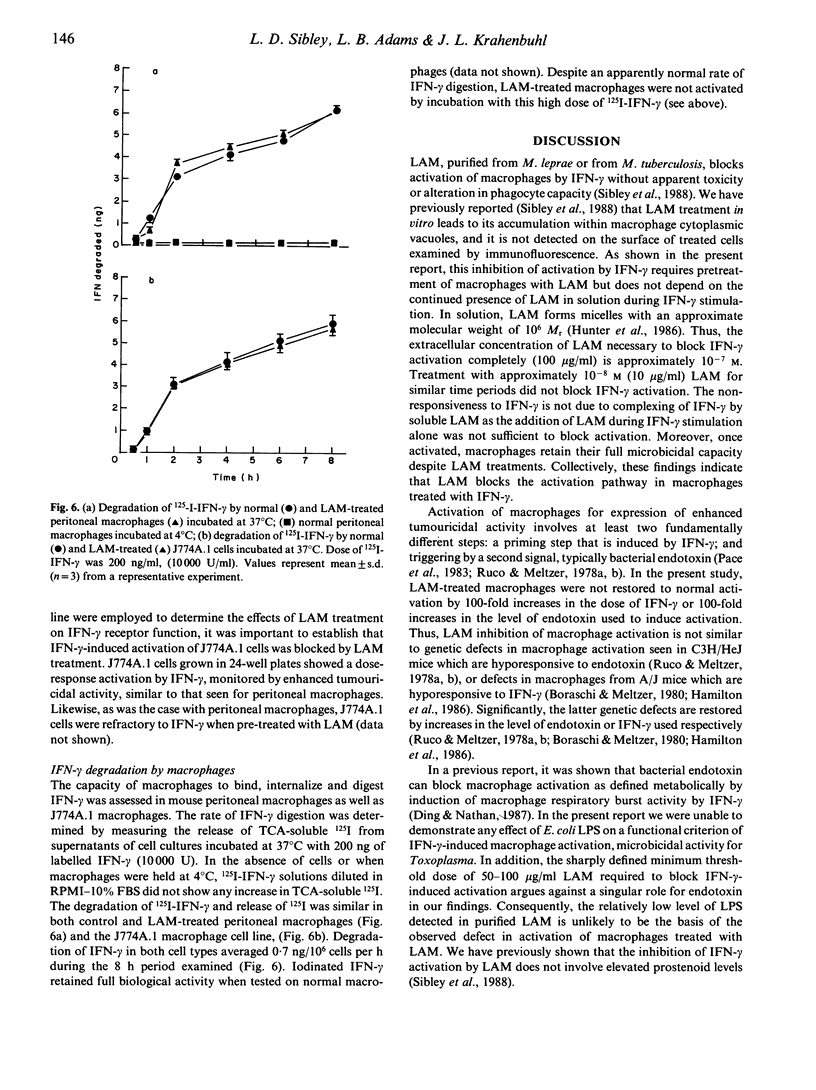
Lipoarabinomannan (LAM), purified from the cell walls of Mycobacterium leprae and M. tuberculosis, is a potent inhibitor of interferon-gamma (IFN-gamma) mediated activation of macrophages. The capability of LAM to inhibit IFN-gamma activation of macrophages in vitro was dose dependent and required a 24-h pre-exposure. Defective activation was evident as a block in IFN-gamma-induced cytocidal activity for tumour cell targets and microbicidal capacity for intracellular Toxoplasma gondii. Additionally, LAM treatment blocked the induction of surface Ia antigens on peritoneal macrophages by IFN-gamma. The requirement for pretreatment with LAM was further substantiated by the finding that peritoneal macrophages that were activated in vivo were not affected by LAM treatments and retained full microbicidal function. However, once inhibited by LAM treatment in vitro, macrophages remained fully refractory to IFN-gamma activation for up to 5 days in culture. Inhibition of IFN-gamma activation in macrophages treated with LAM was not overcome by 100-fold increases in the dose of IFN-gamma used or by a constant dose of IFN-gamma in combination with 100-fold increases in the level of endotoxin used to trigger cytotoxic activity. The defect in IFN-gamma unresponsiveness was not due to altered receptor function, as control and LAM-treated macrophages showed similar capacity to bind, internalize, and digest radiolabelled IFN-gamma. Based on the in vitro findings reported here, the inhibition of IFN-gamma-mediated macrophage activation by exposure to LAM may contribute to defective macrophage function observed in lepromatous granulomas and thus constitutes an important aspect of pathogenesis in mycobacterioses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boraschi D., Meltzer M. S. Defective tumoricidal capacity of macrophages from A/J mice. III. Genetic analysis of the macrophage defect. J Immunol. 1980 Mar;124(3):1050–1053. [PubMed] [Google Scholar]
- Celada A., Allen R., Esparza I., Gray P. W., Schreiber R. D. Demonstration and partial characterization of the interferon-gamma receptor on human mononuclear phagocytes. J Clin Invest. 1985 Dec;76(6):2196–2205. doi: 10.1172/JCI112228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada A., Gray P. W., Rinderknecht E., Schreiber R. D. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984 Jul 1;160(1):55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada A., Schreiber R. D. Internalization and degradation of receptor-bound interferon-gamma by murine macrophages. Demonstration of receptor recycling. J Immunol. 1987 Jul 1;139(1):147–153. [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F. Trace levels of bacterial lipopolysaccharide prevent interferon-gamma or tumor necrosis factor-alpha from enhancing mouse peritoneal macrophage respiratory burst capacity. J Immunol. 1987 Sep 15;139(6):1971–1977. [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Gaylord H., Brennan P. J., Young D. B., Buchanan T. M. Most Mycobacterium leprae carbohydrate-reactive monoclonal antibodies are directed to lipoarabinomannan. Infect Immun. 1987 Nov;55(11):2860–2863. doi: 10.1128/iai.55.11.2860-2863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Somers S. D., Becton D. L., Celada A., Schreiber R. D., Adams D. O. Analysis of deficiencies in IFN-gamma-mediated priming for tumor cytotoxicity in peritoneal macrophages from A/J mice. J Immunol. 1986 Nov 15;137(10):3367–3371. [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. Cytostatic effects of activated macrophages on tumor target cells: inhibition of cytotoxic action of ARA-C. J Immunopharmacol. 1980;2(3):325–348. doi: 10.3109/08923978009046465. [DOI] [PubMed] [Google Scholar]
- Misaki A., Azuma I., Yamamura Y. Structural and immunochemical studies on D-arabino-D-mannans and D-mannans of Mycobacterium tuberculosis and other Mycobacterium species. J Biochem. 1977 Dec;82(6):1759–1770. doi: 10.1093/oxfordjournals.jbchem.a131874. [DOI] [PubMed] [Google Scholar]
- Moreno C., Mehlert A., Lamb J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin Exp Immunol. 1988 Nov;74(2):206–210. [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Gross J. M., Brozna J. P., Goren M. B. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J Immunol. 1988 Jan 15;140(2):634–640. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Defective tumoricidal capacity of macrophages from C3H/HeJ mice. J Immunol. 1978 Jan;120(1):329–334. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: development of macrophage cytotoxic activity requires completion of a sequence of short-lived intermediary reactions. J Immunol. 1978 Nov;121(5):2035–2042. [PubMed] [Google Scholar]
- Sibley L. D., Hunter S. W., Brennan P. J., Krahenbuhl J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988 May;56(5):1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Defective activation of granuloma macrophages from Mycobacterium leprae-infected nude mice. J Leukoc Biol. 1988 Jan;43(1):60–66. doi: 10.1002/jlb.43.1.60. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Mycobacterium leprae-burdened macrophages are refractory to activation by gamma interferon. Infect Immun. 1987 Feb;55(2):446–450. doi: 10.1128/iai.55.2.446-450.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


