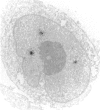
Abstract
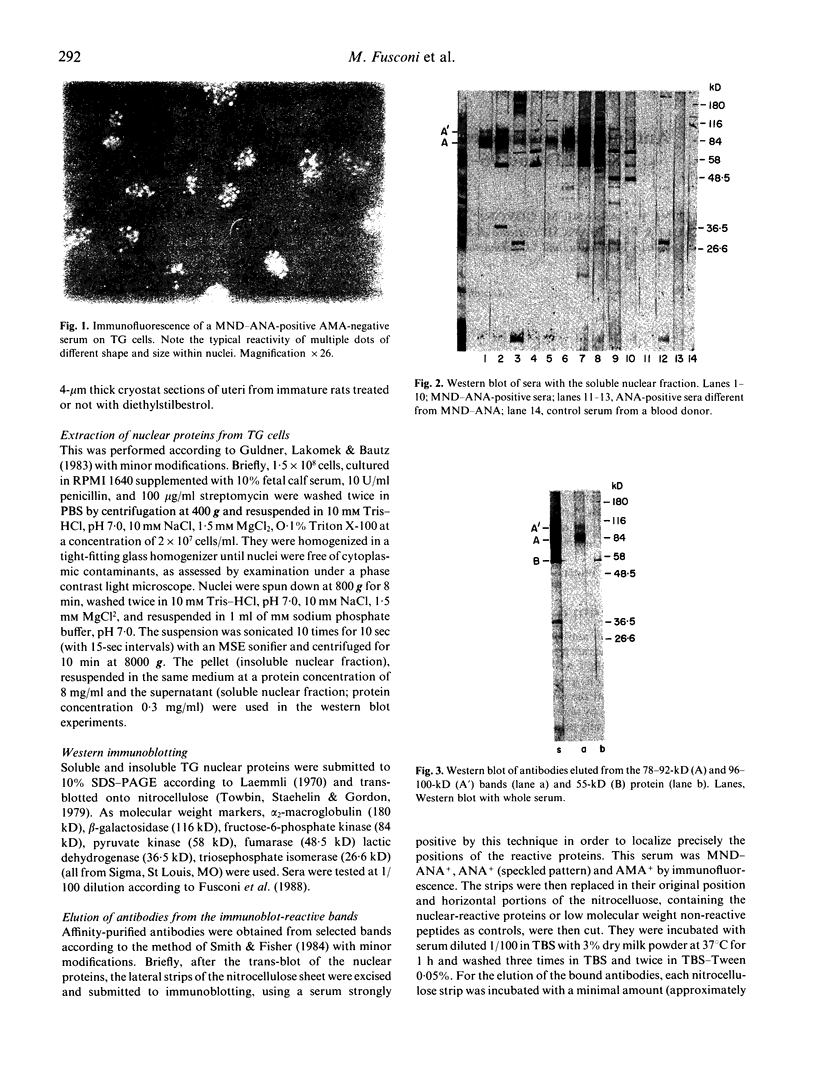
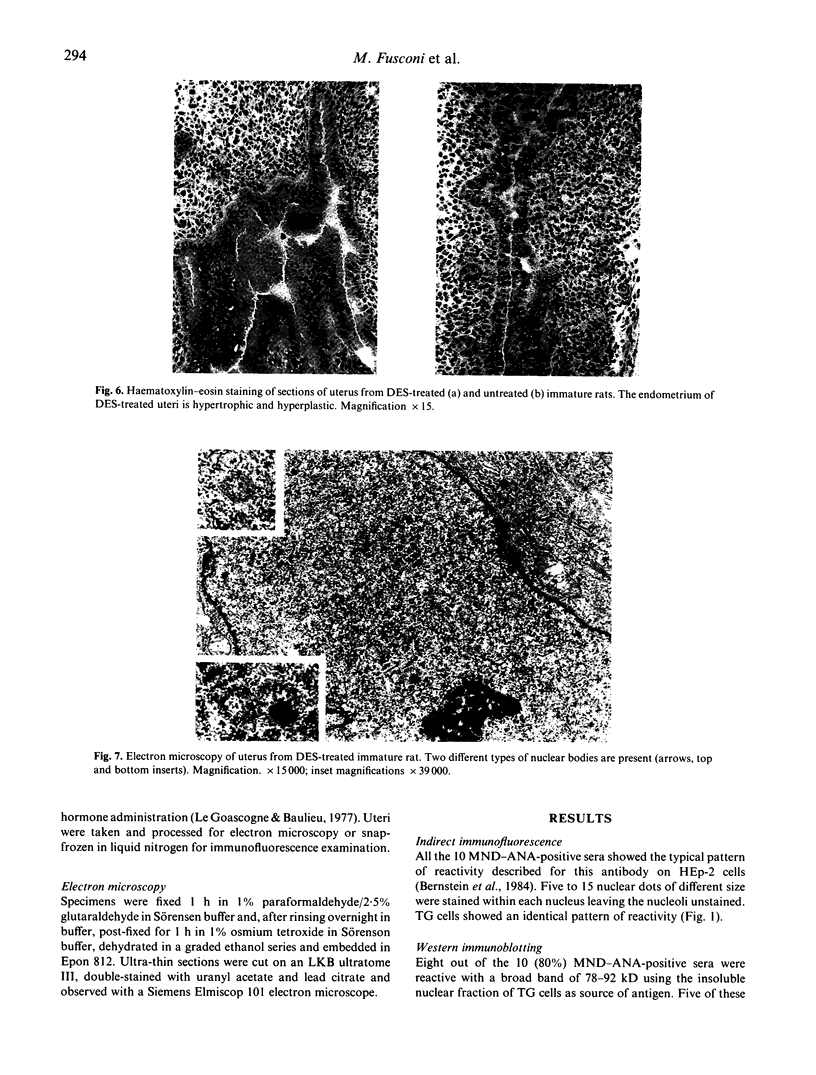
The specificities of anti-nuclear antibodies (ANA) reacting with multiple nuclear dots (MND-ANA) present in about 15% primary biliary cirrhosis sera were studied by Western blot analysis with nuclear fractions from a human cell line. Reactivity with two broad bands of 78-92 kD and 96-100 kD of the insoluble fraction was present exclusively in MND-ANA-positive sera. Antibodies eluted from these proteins specifically retained the immunofluorescence reactivity of MND-ANA. Immunomorphological analysis by a pre-embedding technique revealed that the antibody specifically binds to nuclear regions resembling in size and number nuclear bodies. Since these structures are absent in immature rate endometrial cell and can be induced by diethylstilbestrol, we tested MND-ANA by immunofluorescence on cryostat sections of uteri from hormone-treated and untreated immature rats. A strong reaction of nuclear dots was observed predominantly in endometrial cells of hormone treated rats. We thus conclude that MND-ANA present in primary biliary cirrhosis sera are directed against 78-92-kD and 96-100-kD nuclear proteins located in nuclear bodies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein R. M. Antinuclear antibodies in primary biliary cirrhosis. Lancet. 1984 Mar 3;1(8375):508–508. doi: 10.1016/s0140-6736(84)92869-1. [DOI] [PubMed] [Google Scholar]
- Bernstein R. M., Neuberger J. M., Bunn C. C., Callender M. E., Hughes G. R., Williams R. Diversity of autoantibodies in primary biliary cirrhosis and chronic active hepatitis. Clin Exp Immunol. 1984 Mar;55(3):553–560. [PMC free article] [PubMed] [Google Scholar]
- Cassani F., Bianchi F. B., Lenzi M., Volta U., Pisi E. Immunomorphological characterisation of antinuclear antibodies in chronic liver disease. J Clin Pathol. 1985 Jul;38(7):801–805. doi: 10.1136/jcp.38.7.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. H., Hardin J. W., Padykula H. A., Cardasis C. A. Role of estrogen receptor binding and transcriptional activity in the stimulation of hyperestrogenism and nuclear bodies. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2781–2784. doi: 10.1073/pnas.75.6.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusconi M., Ghadiminejad I., Bianchi F. B., Baum H., Bottazzo G. F., Pisi E. Heterogeneity of antimitochondrial antibodies with the M2-M4 pattern by immunofluorescence as assessed by Western immunoblotting and enzyme linked immunosorbent assay. Gut. 1988 Apr;29(4):440–447. doi: 10.1136/gut.29.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldner H. H., Lakomek H. J., Bautz F. A. Identification of human Sm and (U1) RNP antigens by immunoblotting. J Immunol Methods. 1983 Nov 11;64(1-2):45–59. doi: 10.1016/0022-1759(83)90383-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lozano F., Parés A., Borche L., Plana M., Gallart T., Rodés J., Vives J. Autoantibodies against nuclear envelope-associated proteins in primary biliary cirrhosis. Hepatology. 1988 Jul-Aug;8(4):930–938. doi: 10.1002/hep.1840080438. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostecki C., Krippner H., Penner E., Bautz F. A. Autoimmune sera recognize a 100 kD nuclear protein antigen (sp-100). Clin Exp Immunol. 1987 Apr;68(1):108–116. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]