Abstract
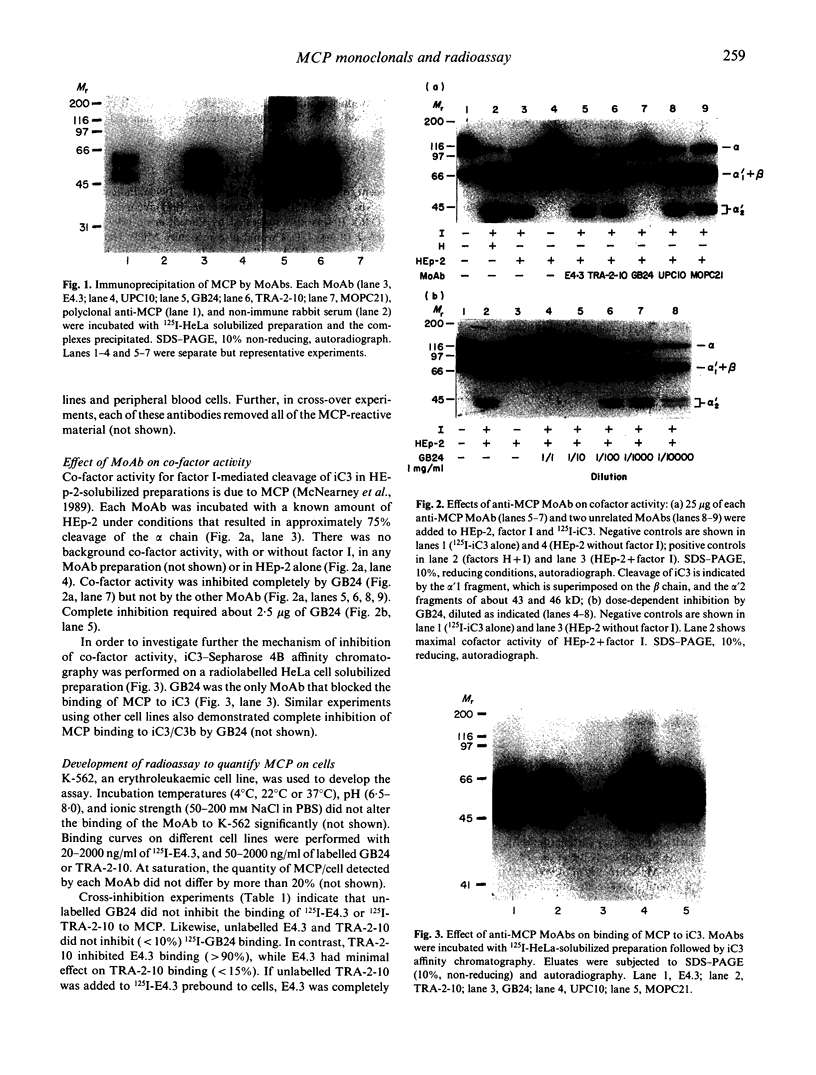
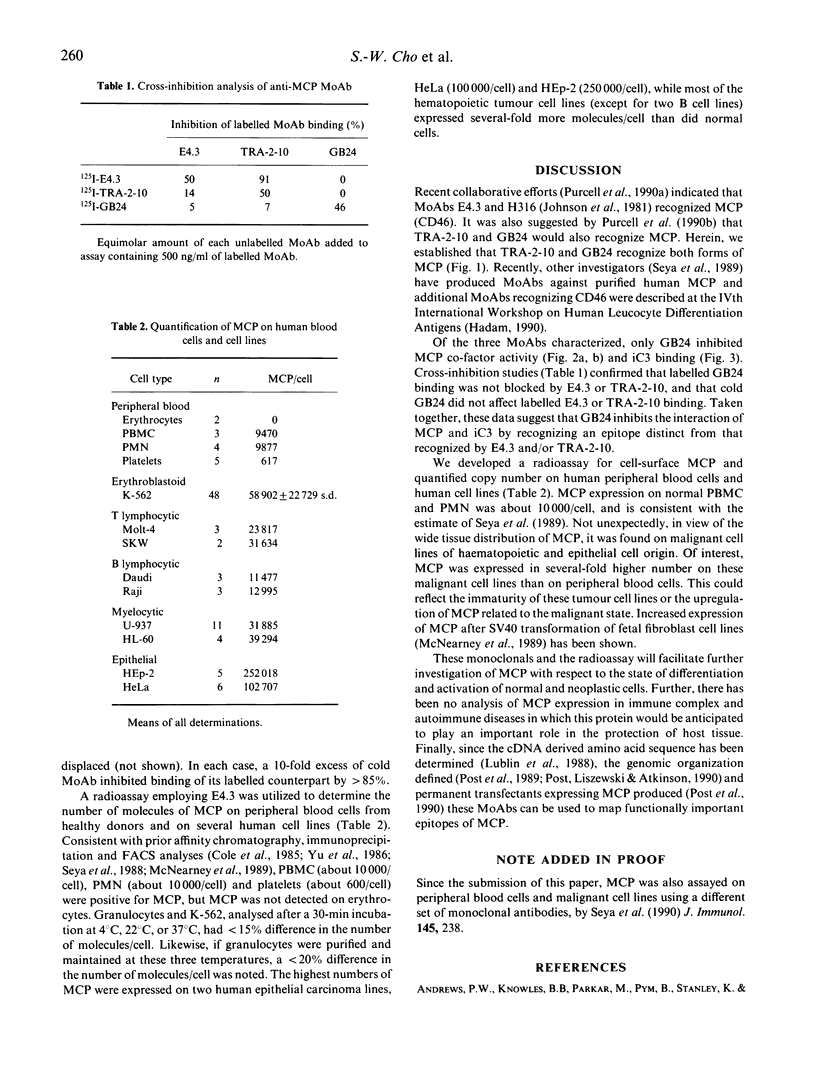
MCP is a widely distributed regulatory glycoprotein of the complement system which binds C3b and C4b and has factor I-dependent co-factor activity. Monoclonal antibodies raised to lymphocytes (E4.3), chorionic microvilli (GB24) and an embryonal carcinoma cell line (TRA-2-10) recognize MCP (CD46). GB24 inhibited both the binding of MCP to its ligand iC3 and co-factor activity; E4.3 and TRA-2-10 did not. The binding of GB24 to cells bearing MCP was not cross-inhibited by E4.3 or TRA-2.10, but TRA-2-10 blocked binding and displaced pre-bound E4.3. Using these antibodies, we developed a radioassay for quantifying the number of MCP molecules/cells. Human peripheral blood mononuclear (PBMC) and polymorphonuclear cells (PMN) had about 10,000 MCP cell; platelets had about 600/cell, and no MCP was found on erythrocytes. Neoplastic hematopoietic cell lines, of myelocytic and T lymphocytic origin, had several-fold more (20-60,000) molecules cell than peripheral blood cells or B cell lines (about 12,000). Malignant epithelial cell lines. HeLa (about 100,000/cell) and HEp-2 (about 250,000 cell) had the highest MCP expression of any cells examined. These monoclonal antibodies--especially GB24, which blocks MCP function--and the direct binding assay will facilitate the further analysis of the biology of this complement regulatory protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W., Knowles B. B., Parkar M., Pym B., Stanley K., Goodfellow P. N. A human cell-surface antigen defined by a monoclonal antibody and controlled by a gene on human chromosome 1. Ann Hum Genet. 1985 Jan;49(Pt 1):31–39. doi: 10.1111/j.1469-1809.1985.tb01673.x. [DOI] [PubMed] [Google Scholar]
- Baenziger N. L., Majerus P. W. Isolation of human platelets and platelet surface membranes. Methods Enzymol. 1974;31:149–155. doi: 10.1016/0076-6879(74)31015-4. [DOI] [PubMed] [Google Scholar]
- Ballard L., Seya T., Teckman J., Lublin D. M., Atkinson J. P. A polymorphism of the complement regulatory protein MCP (membrane cofactor protein or gp45-70). J Immunol. 1987 Jun 1;138(11):3850–3855. [PubMed] [Google Scholar]
- Cole J. L., Housley G. A., Jr, Dykman T. R., MacDermott R. P., Atkinson J. P. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A. 1985 Feb;82(3):859–863. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourcade D., Holers V. M., Atkinson J. P. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- Hsi B. L., Yeh C. J., Fénichel P., Samson M., Grivaux C. Monoclonal antibody GB24 recognizes a trophoblast-lymphocyte cross-reactive antigen. Am J Reprod Immunol Microbiol. 1988 Sep;18(1):21–27. doi: 10.1111/j.1600-0897.1988.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Kulczycki A., Jr, Krause V., Killion C. C., Atkinson J. P. Improved cell surface radioiodination of macrophages. J Immunol Methods. 1980;37(2):133–138. doi: 10.1016/0022-1759(80)90198-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Liszewski M. K., Post T. W., Arce M. A., Le Beau M. M., Rebentisch M. B., Lemons L. S., Seya T., Atkinson J. P. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J Exp Med. 1988 Jul 1;168(1):181–194. doi: 10.1084/jem.168.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNearney T., Ballard L., Seya T., Atkinson J. P. Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J Clin Invest. 1989 Aug;84(2):538–545. doi: 10.1172/JCI114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickells M. W., Atkinson J. P. Characterization of CR1- and membrane cofactor protein-like proteins of two primates. J Immunol. 1990 Jun 1;144(11):4262–4268. [PubMed] [Google Scholar]
- Purcell D. F., McKenzie I. F., Lublin D. M., Johnson P. M., Atkinson J. P., Oglesby T. J., Deacon N. J. The human cell-surface glycoproteins HuLy-m5, membrane co-factor protein (MCP) of the complement system, and trophoblast leucocyte-common (TLX) antigen, are CD46. Immunology. 1990 Jun;70(2):155–161. [PMC free article] [PubMed] [Google Scholar]
- Seya T., Atkinson J. P. Functional properties of membrane cofactor protein of complement. Biochem J. 1989 Dec 1;264(2):581–588. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T., Ballard L. L., Bora N. S., Kumar V., Cui W., Atkinson J. P. Distribution of membrane cofactor protein of complement on human peripheral blood cells. An altered form is found on granulocytes. Eur J Immunol. 1988 Aug;18(8):1289–1294. doi: 10.1002/eji.1830180821. [DOI] [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Akedo H. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J Immunol. 1990 Jul 1;145(1):238–245. [PubMed] [Google Scholar]
- Sparrow R. L., McKenzie I. F. Hu Ly-m5: a unique antigen physically associated with HLA molecules. Hum Immunol. 1983 May;7(1):1–15. doi: 10.1016/0198-8859(83)90002-2. [DOI] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Woof J. M., Burton D. R. Microassay for measurement of binding of radiolabelled ligands to cell surface molecules. J Immunol Methods. 1988 Jul 22;111(2):205–207. doi: 10.1016/0022-1759(88)90128-7. [DOI] [PubMed] [Google Scholar]
- Yu G. H., Holers V. M., Seya T., Ballard L., Atkinson J. P. Identification of a third component of complement-binding glycoprotein of human platelets. J Clin Invest. 1986 Aug;78(2):494–501. doi: 10.1172/JCI112601. [DOI] [PMC free article] [PubMed] [Google Scholar]