Abstract
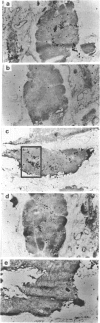
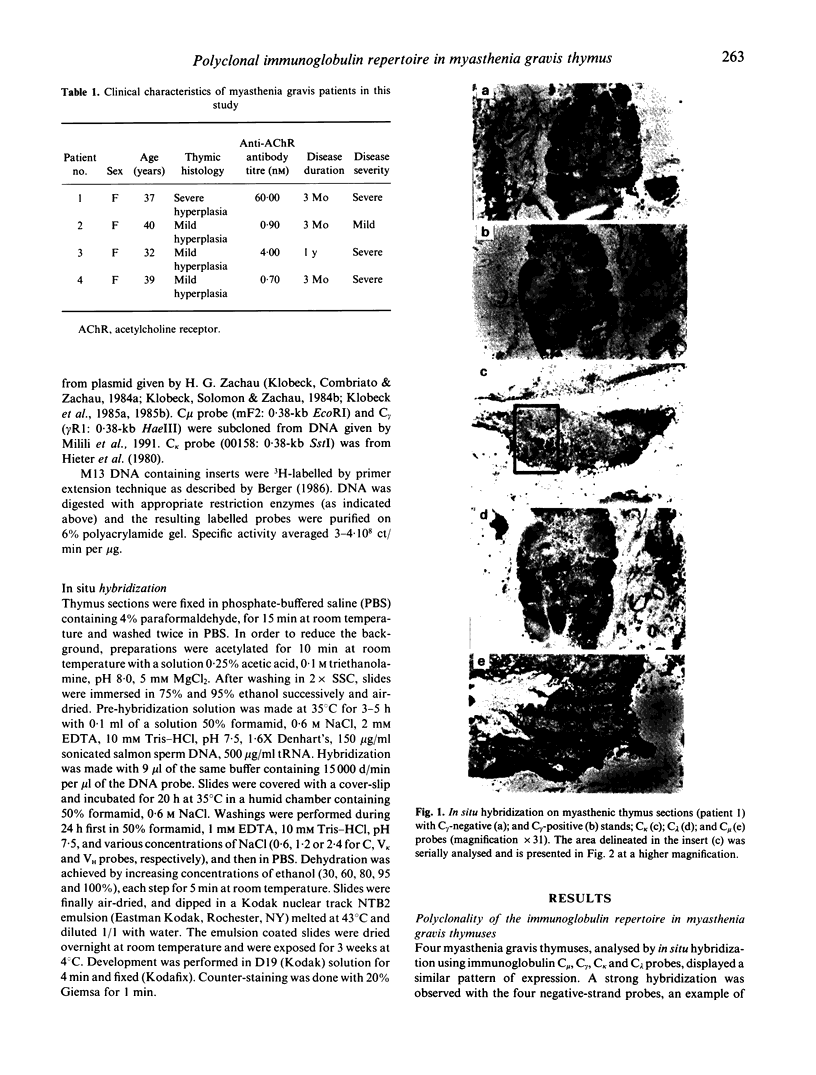
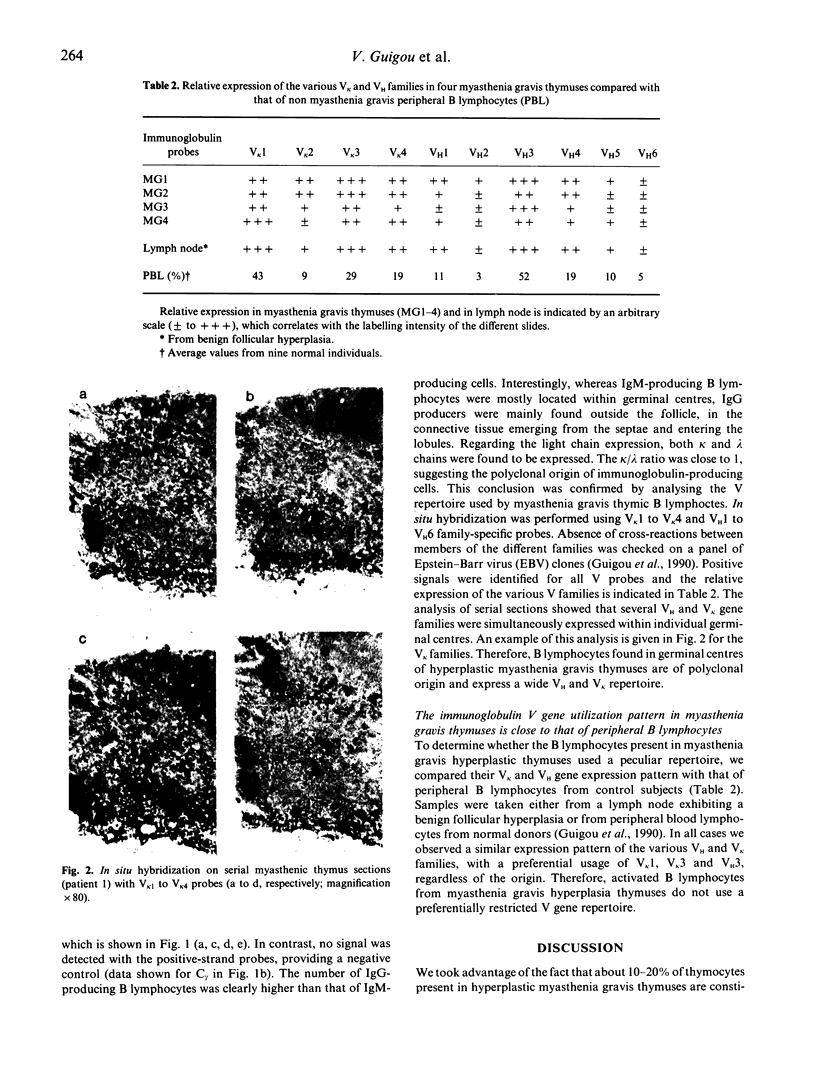
Using in situ hybridization, we analysed the immunoglobulin repertoire expressed by the B cells present in myasthenia gravis thymuses from four patients. B cells, mostly in activated state, were clustered in germinal centres, in which multiple isotypes were identified. A majority of cells expressed IgG as compared with IgM, with a roughly similar contribution of kappa and lambda chains. Hybridization with the six VH and the 4 VK human family probes was observed in serial sections, providing additional evidence that individual germinal centres were polyclonal. The thymic B cell repertoire closely reflected the VH and the VK family usage of normal peripheral blood lymphocytes with the preferential utilization of VH3, VK1 and VK3.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrih-Aknin S., Morel E., Raimond F., Safar D., Gaud C., Binet J. P., Levasseur P., Bach J. F. The role of the thymus in myasthenia gravis: immunohistological and immunological studies in 115 cases. Ann N Y Acad Sci. 1987;505:50–70. doi: 10.1111/j.1749-6632.1987.tb51282.x. [DOI] [PubMed] [Google Scholar]
- Bofill M., Janossy G., Willcox N., Chilosi M., Trejdosiewicz L. K., Newsom-Davis J. Microenvironments in the normal thymus and the thymus in myasthenia gravis. Am J Pathol. 1985 Jun;119(3):462–473. [PMC free article] [PubMed] [Google Scholar]
- Castleman B. The pathology of the thymus gland in myasthenia gravis. Ann N Y Acad Sci. 1966 Jan 26;135(1):496–505. doi: 10.1111/j.1749-6632.1966.tb45497.x. [DOI] [PubMed] [Google Scholar]
- Compston D. A., Vincent A., Newsom-Davis J., Batchelor J. R. Clinical, pathological, HLA antigen and immunological evidence for disease heterogeneity in myasthenia gravis. Brain. 1980 Sep;103(3):579–601. doi: 10.1093/brain/103.3.579. [DOI] [PubMed] [Google Scholar]
- Farrell M. A., Kaufmann J. C., Gilbert J. J., Noseworthy J. H., Armstrong H. A., Ebers G. C. Oligoclonal bands in multiple sclerosis: clinical-pathologic correlation. Neurology. 1985 Feb;35(2):212–218. doi: 10.1212/wnl.35.2.212. [DOI] [PubMed] [Google Scholar]
- Fishleder A., Tubbs R., Hesse B., Levine H. Uniform detection of immunoglobulin-gene rearrangement in benign lymphoepithelial lesions. N Engl J Med. 1987 Apr 30;316(18):1118–1121. doi: 10.1056/NEJM198704303161803. [DOI] [PubMed] [Google Scholar]
- Fujii N., Itoyama Y., Tabira T., Kuroiwa Y. Subsets of lymphoid cells in blood and thymus in myasthenia gravis. Monoclonal antibody analysis. J Neuroimmunol. 1983 Jun;4(3):151–159. doi: 10.1016/0165-5728(83)90031-0. [DOI] [PubMed] [Google Scholar]
- Fujii Y., Hashimoto J., Monden Y., Ito T., Nakahara K., Kawashima Y. Specific activation of lymphocytes against acetylcholine receptor in the thymus in myasthenia gravis. J Immunol. 1986 Feb 1;136(3):887–891. [PubMed] [Google Scholar]
- Guigou V., Cuisinier A. M., Tonnelle C., Moinier D., Fougereau M., Fumoux F. Human immunoglobulin VH and VK repertoire revealed by in situ hybridization. Mol Immunol. 1990 Sep;27(9):935–940. doi: 10.1016/0161-5890(90)90161-r. [DOI] [PubMed] [Google Scholar]
- Heidenreich F., Vincent A., Willcox N., Newsom-Davis J. Anti-acetylcholine receptor antibody specificities in serum and in thymic cell culture supernatants from myasthenia gravis patients. Neurology. 1988 Nov;38(11):1784–1788. doi: 10.1212/wnl.38.11.1784. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Max E. E., Seidman J. G., Maizel J. V., Jr, Leder P. Cloned human and mouse kappa immunoglobulin constant and J region genes conserve homology in functional segments. Cell. 1980 Nov;22(1 Pt 1):197–207. doi: 10.1016/0092-8674(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Isaacson P. G., Norton A. J., Addis B. J. The human thymus contains a novel population of B lymphocytes. Lancet. 1987 Dec 26;2(8574):1488–1491. doi: 10.1016/s0140-6736(87)92622-5. [DOI] [PubMed] [Google Scholar]
- Kimoto H., Shirasawa T., Taniguchi M., Takemori T. B cell precursors are present in the thymus during early development. Eur J Immunol. 1989 Jan;19(1):97–104. doi: 10.1002/eji.1830190116. [DOI] [PubMed] [Google Scholar]
- Klobeck H. G., Bornkamm G. W., Combriato G., Mocikat R., Pohlenz H. D., Zachau H. G. Subgroup IV of human immunoglobulin K light chains is encoded by a single germline gene. Nucleic Acids Res. 1985 Sep 25;13(18):6515–6529. doi: 10.1093/nar/13.18.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Combriato G., Zachau H. G. Immunoglobulin genes of the kappa light chain type from two human lymphoid cell lines are closely related. Nucleic Acids Res. 1984 Sep 25;12(18):6995–7006. doi: 10.1093/nar/12.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Meindl A., Combriato G., Solomon A., Zachau H. G. Human immunoglobulin kappa light chain genes of subgroups II and III. Nucleic Acids Res. 1985 Sep 25;13(18):6499–6513. doi: 10.1093/nar/13.18.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaira M., Kinashi T., Umemura I., Matsuda F., Noma T., Ono Y., Honjo T. Organization and evolution of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1986 Aug 20;190(4):529–541. doi: 10.1016/0022-2836(86)90239-1. [DOI] [PubMed] [Google Scholar]
- Levine G. D., Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol. 1978 Sep;9(5):495–515. doi: 10.1016/s0046-8177(78)80131-2. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Seybold M. E., Lennon V. A., Whittingham S., Duane D. D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976 Nov;26(11):1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- Miyama-Inaba M., Kuma S., Inaba K., Ogata H., Iwai H., Yasumizu R., Muramatsu S., Steinman R. M., Ikehara S. Unusual phenotype of B cells in the thymus of normal mice. J Exp Med. 1988 Aug 1;168(2):811–816. doi: 10.1084/jem.168.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch H., Hohlfeld R., Toyka K. V. Analysis of immunoglobulin and T cell receptor gene rearrangements in the thymus of myasthenia gravis patients. J Neuroimmunol. 1989 Feb;21(2-3):169–176. doi: 10.1016/0165-5728(89)90172-0. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Kokla A., Walgrave S. L., Conti-Tronconi B. M. Localization of the main immunogenic region of human muscle acetylcholine receptor to residues 67-76 of the alpha subunit. Proc Natl Acad Sci U S A. 1988 May;85(9):2899–2903. doi: 10.1073/pnas.85.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Seybold M. E., Lindstrom J. M. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Jan;79(1):188–192. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Whiting P. J., Schluep M., Heidenreich F., Lang B., Roberts A., Willcox N., Newsom-Davis J. Antibody heterogeneity and specificity in myasthenia gravis. Ann N Y Acad Sci. 1987;505:106–120. doi: 10.1111/j.1749-6632.1987.tb51286.x. [DOI] [PubMed] [Google Scholar]
- Williams C. L., Lennon V. A. Thymic B lymphocyte clones from patients with myasthenia gravis secrete monoclonal striational autoantibodies reacting with myosin, alpha actinin, or actin. J Exp Med. 1986 Oct 1;164(4):1043–1059. doi: 10.1084/jem.164.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]