Abstract
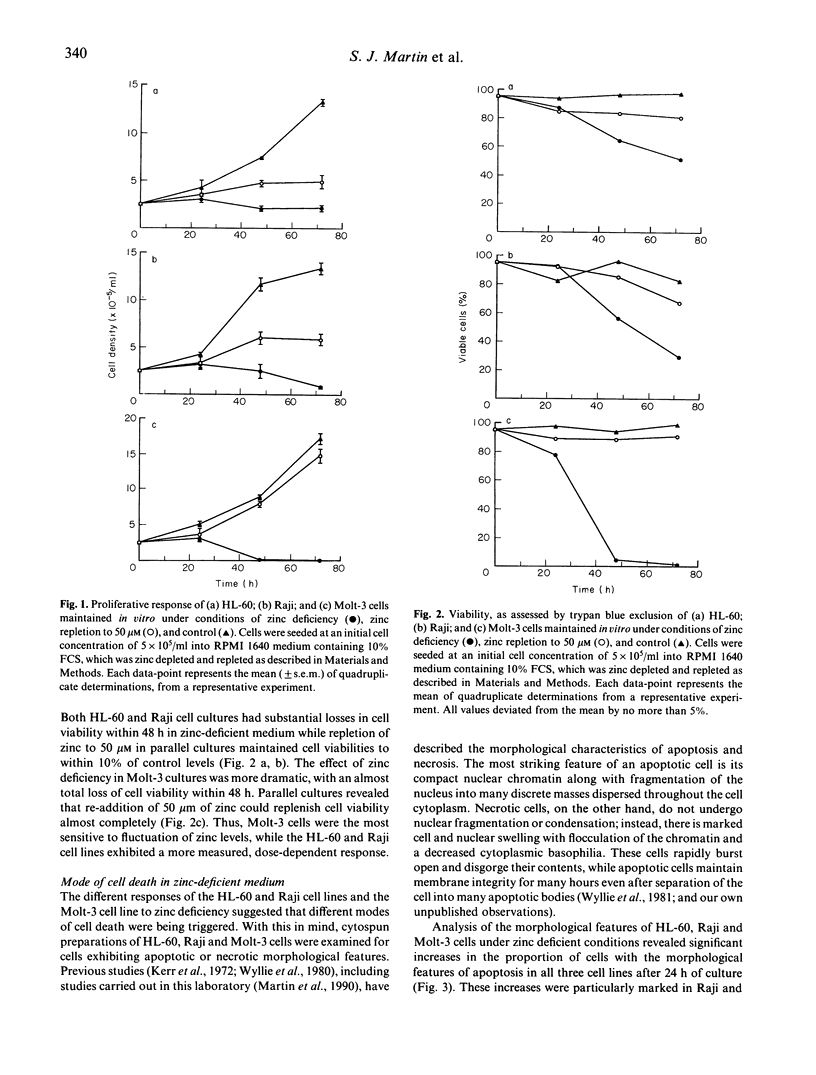
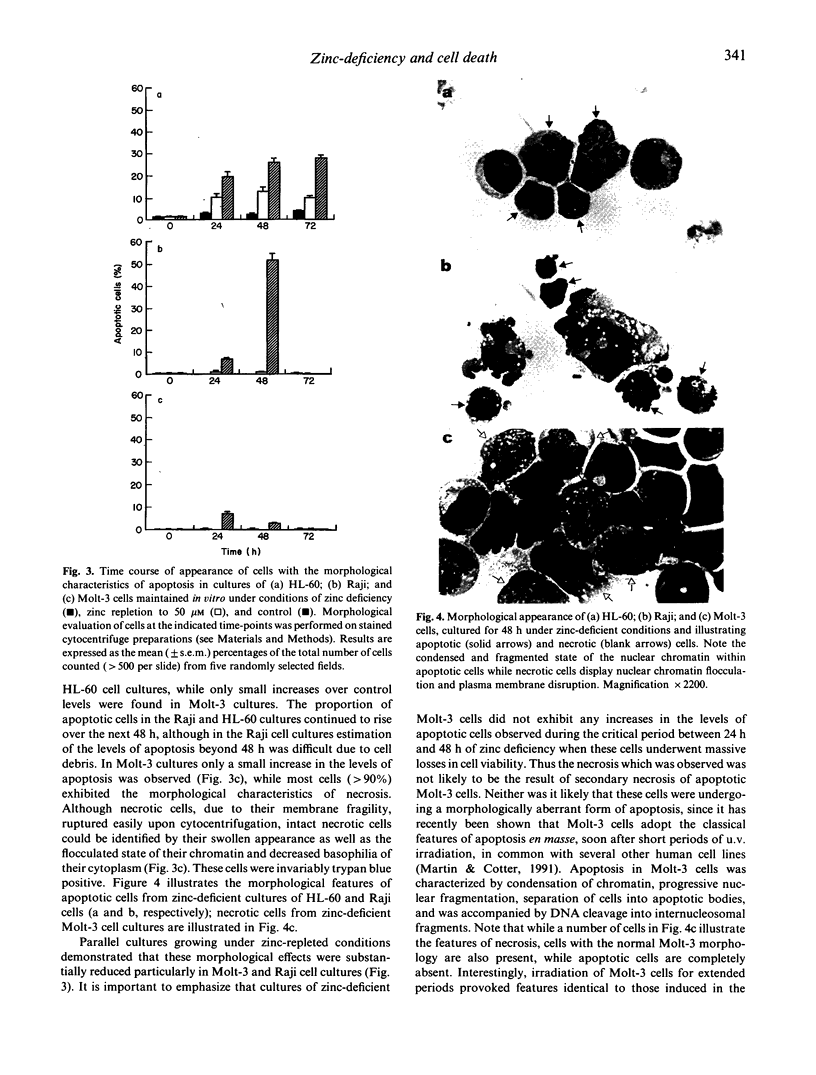
Three human cell lines of lymphoid (Molt-3 and Raji) or myeloid (HL-60) origin were maintained in vitro under zinc-sufficient or zinc-deficient conditions. Under these conditions, cell proliferation, viability and mode of death (apoptotic or necrotic) were assessed. All three cell types decreased their proliferative capacity and viability under conditions of zinc deficiency. Cell death in the HL-60 and Raji cultures occurred primarily via apoptosis, while most cells in zinc-deficient Molt-3 cultures died via necrosis. Apoptosis in zinc-deficient cultures of HL-60 and Raji cells was characterized by a slow decline in culture viability as cells with condensed and fragmented nuclear DNA appeared. These morphological changes were accompanied by an increase in cell buoyant density, which allowed separation of viable apoptotic cells from their non-apoptotic counterparts by means of percoll stepdensity gradients. Necrosis in zinc-deficient Molt-3 cultures was characterized by rapid loss of cell culture viability as these cells underwent direct lysis. Intact necrotic cells were easily identified by the flocculated state of their chromatin as well as the decreased basophilia of their cytoplasm. Analysis of DNA from apoptotic HL-60 and Raji cells revealed that internucleosomal DNA degradation, indicative of endogenous endonuclease activation, had occurred, whereas the nuclear DNA of necrotic Molt-3 cells remained relatively unfragmented. The different modes of cell death evoked may reflect the relative sensitivities of cells of these lineages to zinc levels in vivo.
Full text
PDF



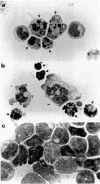
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen J. J., Duke R. C., Chervenak R., Sellins K. S., Olson L. K. DNA fragmentation in targets of CTL: an example of programmed cell death in the immune system. Adv Exp Med Biol. 1985;184:493–508. doi: 10.1007/978-1-4684-8326-0_32. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Cossack Z. T. T-lymphocyte dysfunction in the elderly associated with zinc deficiency and subnormal nucleoside phosphorylase activity: effect of zinc supplementation. Eur J Cancer Clin Oncol. 1989 Jun;25(6):973–976. doi: 10.1016/0277-5379(89)90156-9. [DOI] [PubMed] [Google Scholar]
- Dowd P. S., Kelleher J., Guillou P. J. T-lymphocyte subsets and interleukin-2 production in zinc-deficient rats. Br J Nutr. 1986 Jan;55(1):59–69. doi: 10.1079/bjn19860010. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN M. A., BARR Y. M. CULTIVATION IN VITRO OF HUMAN LYMPHOBLASTS FROM BURKITT'S MALIGNANT LYMPHOMA. Lancet. 1964 Feb 1;1(7327):252–253. doi: 10.1016/s0140-6736(64)92354-2. [DOI] [PubMed] [Google Scholar]
- Elmes M. E. Apoptosis in the small intestine of zinc-deficient and fasted rats. J Pathol. 1977 Dec;123(4):219–223. doi: 10.1002/path.1711230404. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Nair M., Onoe K., Tanaka T., Floyd R., Good R. A. Impairment of cell-mediated immunity functions by dietary zinc deficiency in mice. Proc Natl Acad Sci U S A. 1979 Jan;76(1):457–461. doi: 10.1073/pnas.76.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., DePasquale-Jardieu P., Zwickl C. M., Luecke R. W. Regeneration of T-cell helper function in zinc-deficient adult mice. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5660–5664. doi: 10.1073/pnas.75.11.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Haas S. M., Luecke R. W. Effect of zinc deficiency on the immune response of the young adult A/J mouse. J Nutr. 1977 Oct;107(10):1889–1895. doi: 10.1093/jn/107.10.1889. [DOI] [PubMed] [Google Scholar]
- Hunt J. B., Rhee M. J., Storm C. B. A rapid and convenient preparation of apocarbonic anhydrase. Anal Biochem. 1977 May 1;79(1-2):614–617. doi: 10.1016/0003-2697(77)90444-4. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. P., Nawaz S., Gerschenson L. E. Evidence for soluble factors regulating cell death and cell proliferation in primary cultures of rabbit endometrial cells grown on collagen. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4784–4788. doi: 10.1073/pnas.83.13.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavé I., Rondón Benaím I. Modulatory effect of zinc on the proliferative response of murine spleen cells to polyclonal T cell mitogens. Cell Immunol. 1984 Dec;89(2):322–330. doi: 10.1016/0008-8749(84)90334-4. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Bradley J. G., Cotter T. G. HL-60 cells induced to differentiate towards neutrophils subsequently die via apoptosis. Clin Exp Immunol. 1990 Mar;79(3):448–453. doi: 10.1111/j.1365-2249.1990.tb08110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J., Lennon S. V., Bonham A. M., Cotter T. G. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990 Sep 15;145(6):1859–1867. [PubMed] [Google Scholar]
- Mathe G., Blazsek I., Gil-Delgado M. A., Canon C., Misset J. L., Gaget H., Reizenstein P. The effect of zinc on normal and neoplastic T-lymphocyte proliferation. Med Oncol Tumor Pharmacother. 1985;2(3):203–210. doi: 10.1007/BF02934549. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Moulder K., Steward M. W. Experimental zinc deficiency: effects on cellular responses and the affinity of humoral antibody. Clin Exp Immunol. 1989 Aug;77(2):269–274. [PMC free article] [PubMed] [Google Scholar]
- Oleske J. M., Westphal M. L., Shore S., Gorden D., Bogden J. D., Nahmias A. Zinc therapy of depressed cellular immunity in acrodermatitis enteropathica. Its correction. Am J Dis Child. 1979 Sep;133(9):915–918. doi: 10.1001/archpedi.1979.02130090043007. [DOI] [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987 Nov 15;139(10):3199–3206. [PubMed] [Google Scholar]
- Walker N. I., Harmon B. V., Gobé G. C., Kerr J. F. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- Winchurch R. A. Activation of thymocyte responses to interleukin-1 by zinc. Clin Immunol Immunopathol. 1988 May;47(2):174–180. doi: 10.1016/0090-1229(88)90070-0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Yamada T., Ohyama H. Radiation-induced interphase death of rat thymocytes is internally programmed (apoptosis). Int J Radiat Biol Relat Stud Phys Chem Med. 1988 Jan;53(1):65–75. doi: 10.1080/09553008814550431. [DOI] [PubMed] [Google Scholar]
- Zanzonico P., Fernandes G., Good R. A. The differential sensitivity of T-cell and B-cell mitogenesis to in vitro zinc deficiency. Cell Immunol. 1981 May 1;60(1):203–211. doi: 10.1016/0008-8749(81)90260-4. [DOI] [PubMed] [Google Scholar]