Abstract
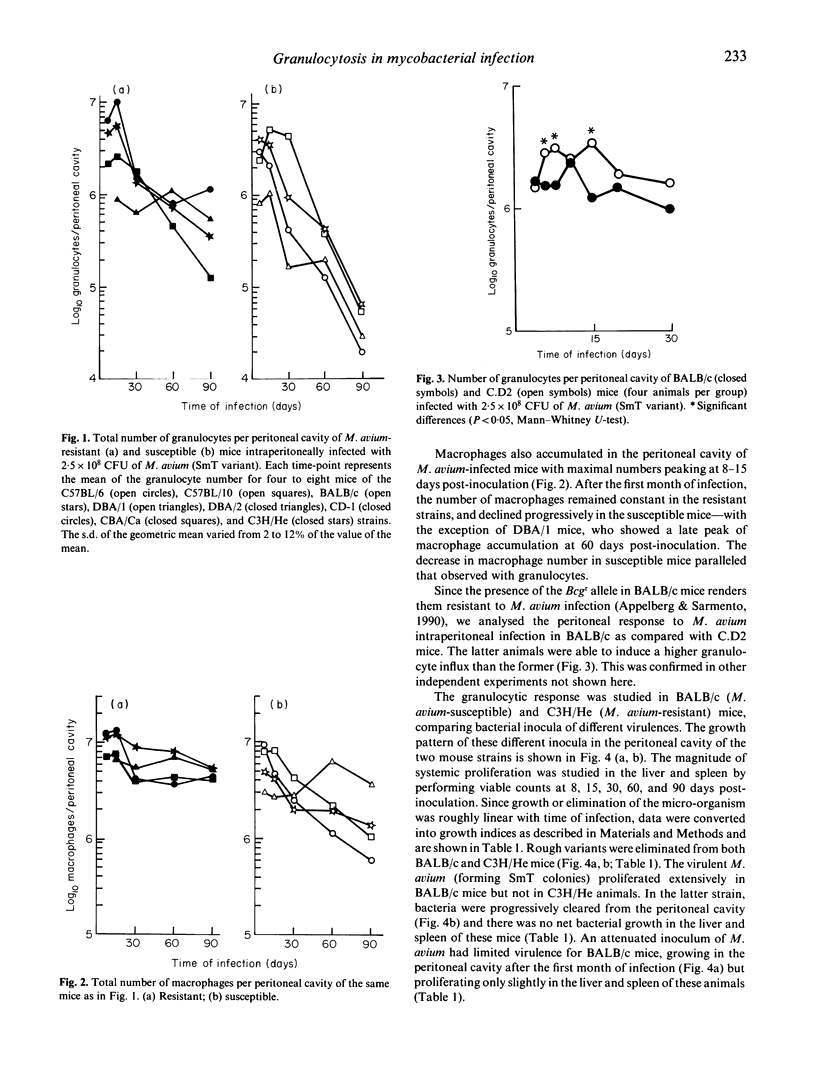
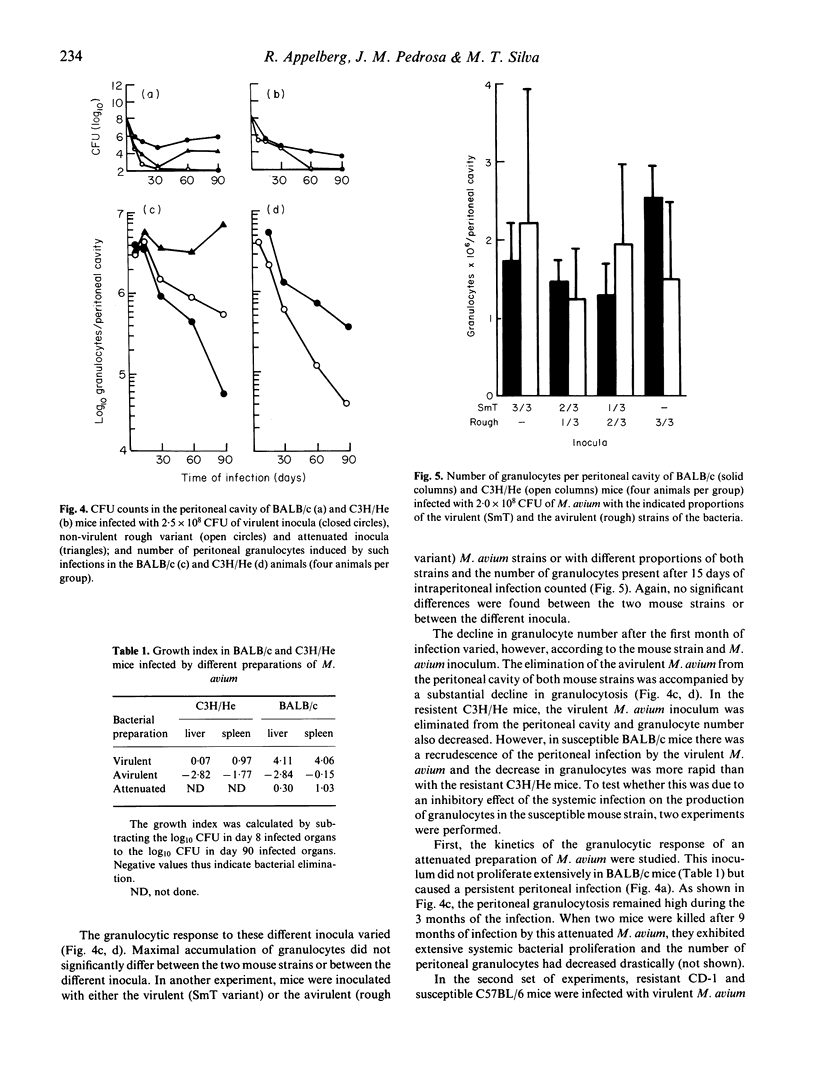
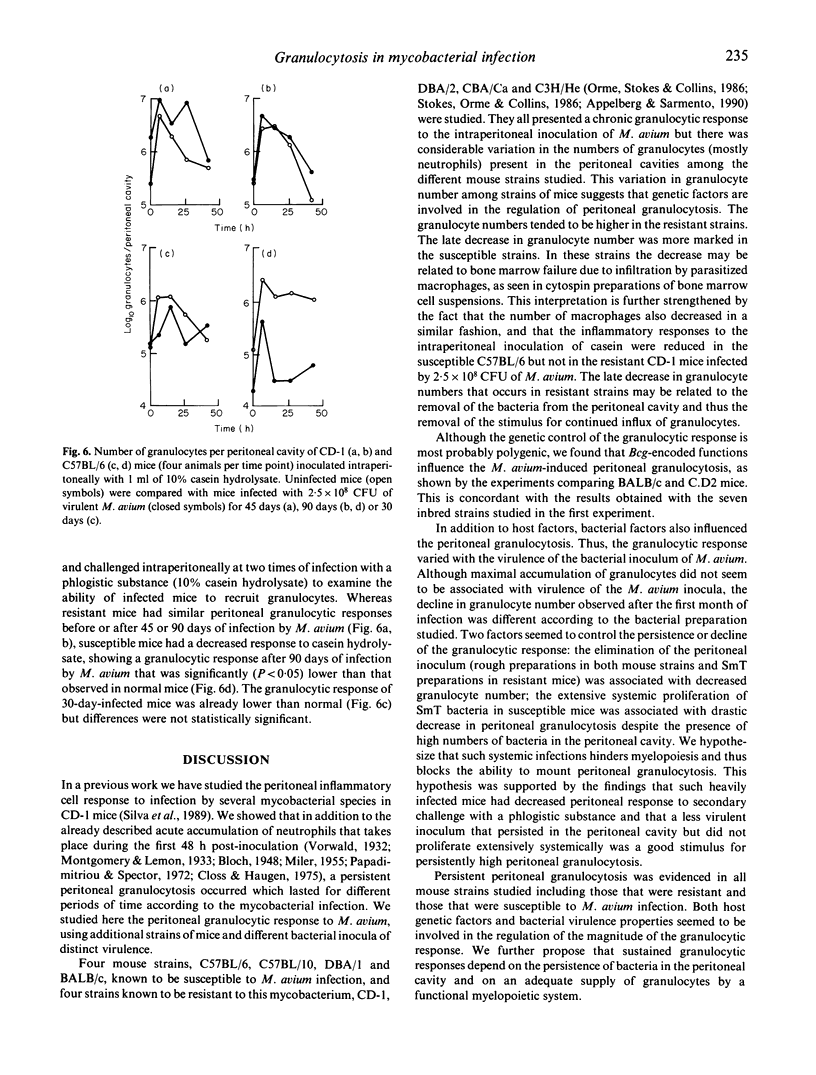
Persistent peritoneal granulocytosis and elevated macrophage counts have been found in nine mouse strains from 8 to 90 days after infection with Mycobacterium avium. Peritoneal granulocytosis was higher in M. avium-resistant BALB/c. Bcgr (C.D2) mice, compared with congenic M. avium-susceptible BALB/c (Bcgs) animals. Although maximal granulocytosis values were not related to virulence of the inocula, the kinetics of the granulocytic response varied with the virulence of M. avium. Following infections by avirulent (rough) strains of M. avium, the peritoneal granulocytosis progressively declined in BALB/c and C3H/He mice. A similar decline in granulocyte number was observed in resistant C3H/He mice infected with virulent M. avium (smooth transparent strain). In both instances the decline in the peritoneal granulocytosis was associated with a progressive elimination of the inoculum. In the susceptible BALB/c mice, virulent M. avium strains induced progressive infection accompanied with a rapid decline in granulocyte number, whereas the infection with attenuated M. avium, which caused a chronic infection, induced persistent granulocytosis. The ability to recruit granulocytes following the intraperitoneal inoculation of a phlogistic substance (casein hydrolysate) was decreased in infected susceptible but not in infected resistant mice at 90 days of infection with virulent M. avium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Sarmento A. M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990 Jun;80(3):324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Silva M. T. T cell-dependent chronic neutrophilia during mycobacterial infections. Clin Exp Immunol. 1989 Dec;78(3):478–483. [PMC free article] [PubMed] [Google Scholar]
- Appelberg R., Soares R., Ferreira P., Silva M. T. Induction of non-specific immunosuppression in mice by mycobacterial infections and its relationship to macrophage activation. Scand J Immunol. 1989 Aug;30(2):165–174. doi: 10.1111/j.1365-3083.1989.tb01198.x. [DOI] [PubMed] [Google Scholar]
- Edwards D., Kirkpatrick C. H. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986 Nov;134(5):1062–1071. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- Heifets L., Imai K., Goren M. B. Expression of peroxidase-dependent iodination by macrophages ingesting neutrophil debris. J Reticuloendothel Soc. 1980 Oct;28(4):391–404. [PubMed] [Google Scholar]
- Lima M. F., Kierszenbaum F. Lactoferrin effects on phagocytic cell function. I. Increased uptake and killing of an intracellular parasite by murine macrophages and human monocytes. J Immunol. 1985 Jun;134(6):4176–4183. [PubMed] [Google Scholar]
- May M. E., Spagnuolo P. J. Evidence for activation of a respiratory burst in the interaction of human neutrophils with Mycobacterium tuberculosis. Infect Immun. 1987 Sep;55(9):2304–2307. doi: 10.1128/iai.55.9.2304-2307.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Stokes R. W., Collins F. M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986 Oct;54(1):56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., Spector W. G. The ultrastructure of high- and low-turnover inflammatory granulomata. J Pathol. 1972 Jan;106(1):37–43. doi: 10.1002/path.1711060104. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Silva M. N., Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989 May;6(5):369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- Smith C. C., Barr R. M., Alexander J. Studies on the interaction of Mycobacterium microti and Mycobacterium lepraemurium with mouse polymorphonuclear leucocytes. J Gen Microbiol. 1979 May;112(1):185–189. doi: 10.1099/00221287-112-1-185. [DOI] [PubMed] [Google Scholar]
- Stokes R. W., Orme I. M., Collins F. M. Role of mononuclear phagocytes in expression of resistance and susceptibility to Mycobacterium avium infections in mice. Infect Immun. 1986 Dec;54(3):811–819. doi: 10.1128/iai.54.3.811-819.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


