Abstract
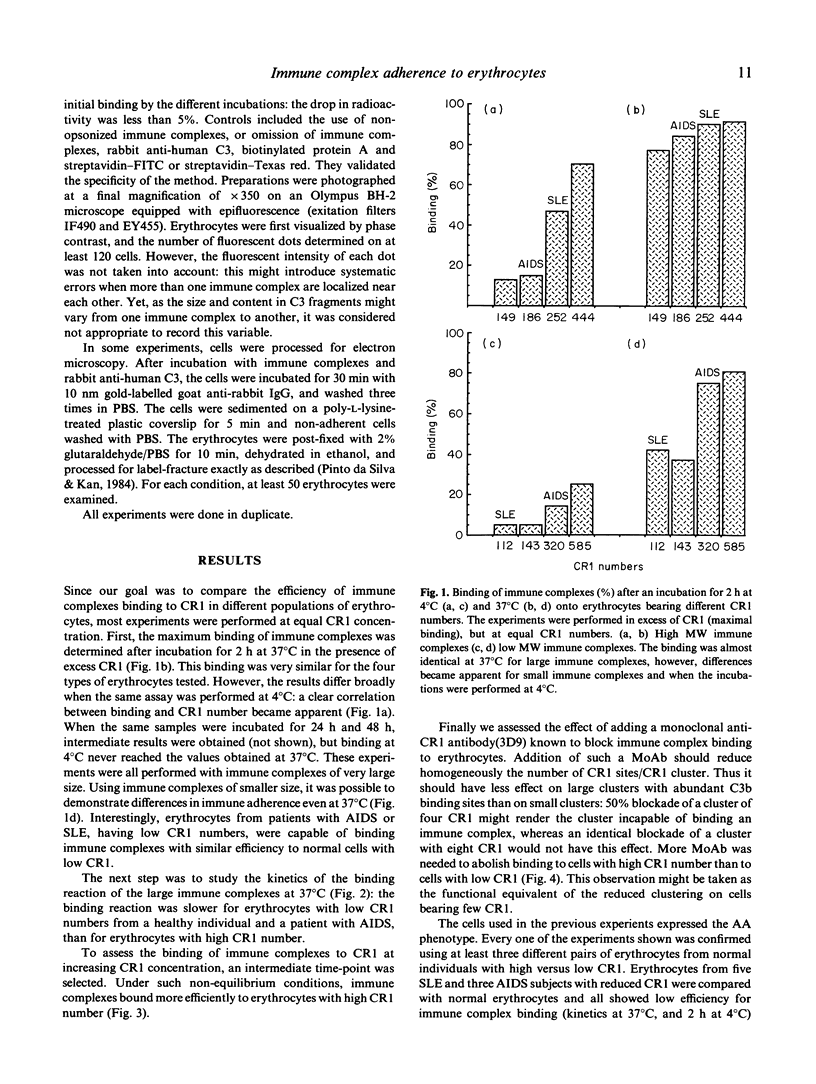
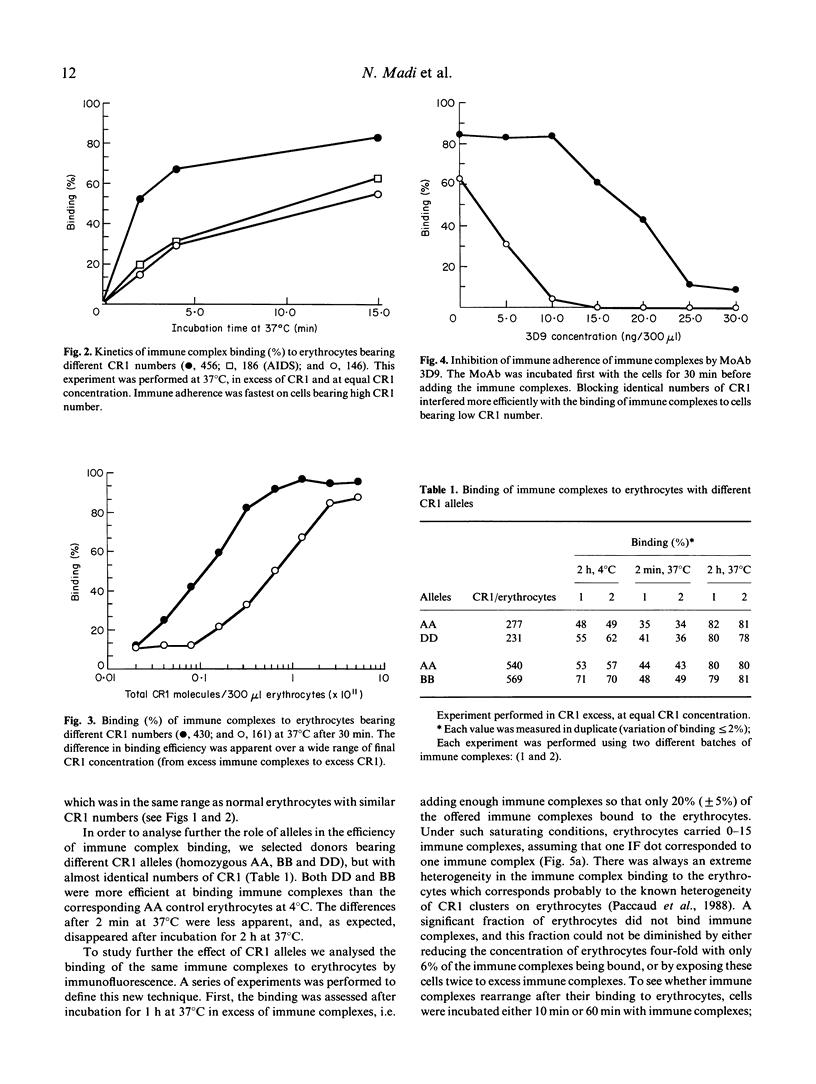
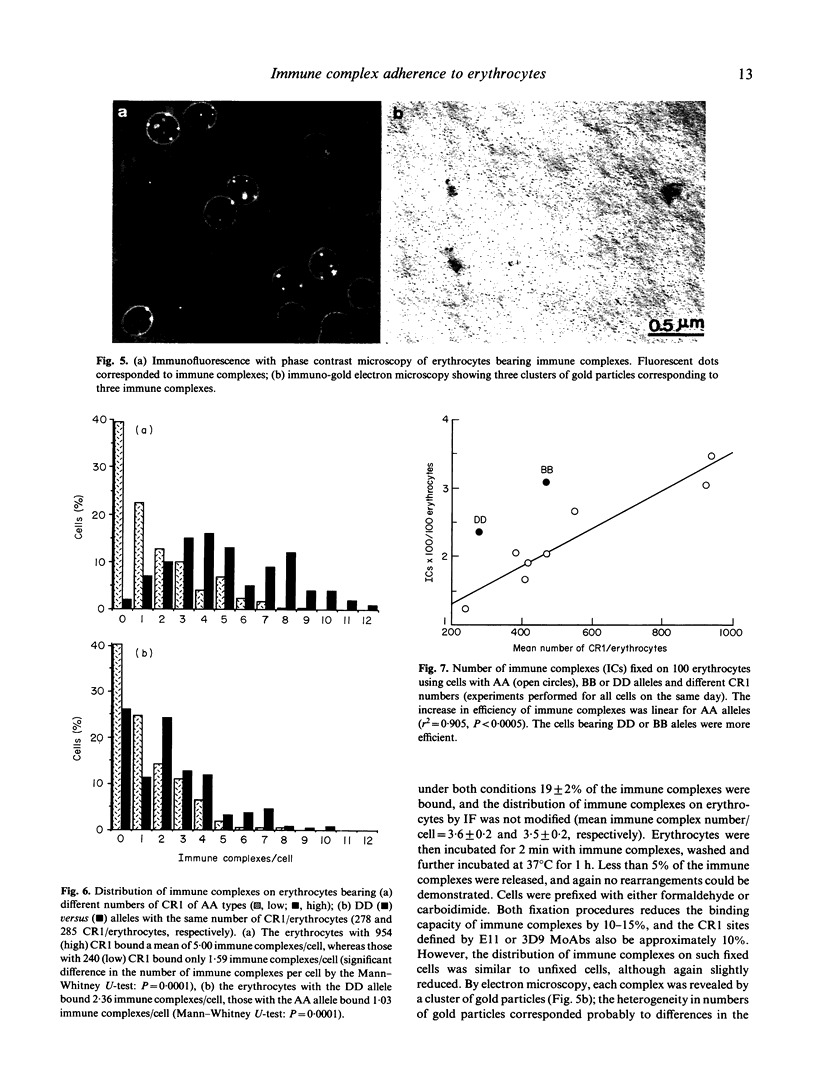
C3b-coated immune complexes adhere to the complement receptor 1 (CR1, CD35) on human erythrocytes. This multi-valent binding might be favoured by the known clustering of CR1 and by the multiple C3b-binding sites on each CR1. The size of the CR1 clusters correlates directly with the number of CR1/erythrocytes, and the different structural CR1 alleles bear between two and five C3b-binding sites. Using radiolabelled hepatitis B surface antigen-antibody complexes, we investigated whether CR1 numbers and structural alleles modulate the ability of erythrocytes to bind immune complexes, and assessed if any reorganization of immune complexes takes place at the erythrocyte surface after the initial binding reaction. The binding efficiency (immune complexes/CR1) correlated with CR1 number as determined by the maximal binding at 4 degrees C, the kinetics of binding at 37 degrees C, and the binding in the presence of excess immune complexes and of immune complexes of small size. Binding efficiencies were similar for erythrocytes with low CR1 from normal subjects and patients with AIDS or SLE. A monoclonal antibody blocking the C3b-binding sites (3D9) of CR1 interfered with binding efficiency at a lower concentration on cells bearing low CR1 numbers, suggesting that CR1 clustering is essential. The larger alleles of CR1 (DD and BB) were more efficient than AA alleles. The distribution of immune complexes, visualized by immunofluorescence, was heterogeneous on erythrocytes: about two out of three cells bore between one and 12 immune complexes. No visible immune complex reorganization took place after initial binding, as prefixed erythrocytes displayed the same immune complex distribution and number/erythrocytes as unfixed erythrocytes. The contribution of CR1 alleles in immune complex binding efficiency was confirmed by morphological analysis. These results demonstrate that immune adherence efficiency is the resultant of the CR1 clustering, as well as the particular alleles carried by erythrocytes. Moreover, there is little or no immune complexes surface reorganization after the initial binding reaction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Melamed J., Tack B. F., Colten H. R. Characterization of the human complement (c3b) receptor with a fluid phase C3b dimer. J Immunol. 1981 Oct;127(4):1348–1354. [PubMed] [Google Scholar]
- Cornacoff J. B., Hebert L. A., Smead W. L., VanAman M. E., Birmingham D. J., Waxman F. J. Primate erythrocyte-immune complex-clearing mechanism. J Clin Invest. 1983 Feb;71(2):236–247. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio F. G., Shen X. P., Hebert L. A. Immune complexes bind preferentially to specific subpopulations of human erythrocytes. Clin Immunol Immunopathol. 1990 Jun;55(3):337–354. doi: 10.1016/0090-1229(90)90123-8. [DOI] [PubMed] [Google Scholar]
- Edberg J. C., Tosic L., Wright E. L., Sutherland W. M., Taylor R. P. Quantitative analyses of the relationship between C3 consumption, C3b capture, and immune adherence of complement-fixing antibody/DNA immune complexes. J Immunol. 1988 Dec 15;141(12):4258–4265. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Lennek R., Baldwin A. S., Jr, Waller S. J., Morley K. W., Taylor R. P. Studies of the physical biochemistry and complement-fixing properties of DNA/anti-DNA immune complexes. J Immunol. 1981 Aug;127(2):602–608. [PubMed] [Google Scholar]
- Medof M. E., Iida K., Mold C., Nussenzweig V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J Exp Med. 1982 Dec 1;156(6):1739–1754. doi: 10.1084/jem.156.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. A., Sim E. Size polymorphism of the erythrocyte complement receptor type 1 (CR1) in systemic lupus erythematosus induced by hydralazine. Complement Inflamm. 1989;6(2):88–93. doi: 10.1159/000463079. [DOI] [PubMed] [Google Scholar]
- NELSON R. A., Jr The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953 Dec 18;118(3077):733–737. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- Ng Y. C., Schifferli J. A., Walport M. J. Immune complexes and erythrocyte CR1 (complement receptor type 1): effect of CR1 numbers on binding and release reactions. Clin Exp Immunol. 1988 Mar;71(3):481–485. [PMC free article] [PubMed] [Google Scholar]
- Paccaud J. P., Carpentier J. L., Schifferli J. A. Difference in the clustering of complement receptor type 1 (CR1) on polymorphonuclear leukocytes and erythrocytes: effect on immune adherence. Eur J Immunol. 1990 Feb;20(2):283–289. doi: 10.1002/eji.1830200209. [DOI] [PubMed] [Google Scholar]
- Paccaud J. P., Carpentier J. L., Schifferli J. A. Direct evidence for the clustered nature of complement receptors type 1 on the erythrocyte membrane. J Immunol. 1988 Dec 1;141(11):3889–3894. [PubMed] [Google Scholar]
- Pinto da Silva P., Kan F. W. Label-fracture: a method for high resolution labeling of cell surfaces. J Cell Biol. 1984 Sep;99(3):1156–1161. doi: 10.1083/jcb.99.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Yount W. J., Walport M. J., Winfield J. B., Parker C. J., Fuller C. R., Taylor R. P., Myones B. L., Lachmann P. J. Disease-associated loss of erythrocyte complement receptors (CR1, C3b receptors) in patients with systemic lupus erythematosus and other diseases involving autoantibodies and/or complement activation. J Immunol. 1985 Sep;135(3):2005–2014. [PubMed] [Google Scholar]
- Schifferli J. A., Ng Y. C., Estreicher J., Walport M. J. The clearance of tetanus toxoid/anti-tetanus toxoid immune complexes from the circulation of humans. Complement- and erythrocyte complement receptor 1-dependent mechanisms. J Immunol. 1988 Feb 1;140(3):899–904. [PubMed] [Google Scholar]
- Schifferli J. A., Ng Y. C., Paccaud J. P., Walport M. J. The role of hypocomplementaemia and low erythrocyte complement receptor type 1 numbers in determining abnormal immune complex clearance in humans. Clin Exp Immunol. 1989 Mar;75(3):329–335. [PMC free article] [PubMed] [Google Scholar]
- Tausk F. A., McCutchan A., Spechko P., Schreiber R. D., Gigli I. Altered erythrocyte C3b receptor expression, immune complexes, and complement activation in homosexual men in varying risk groups for acquired immune deficiency syndrome. J Clin Invest. 1986 Oct;78(4):977–982. doi: 10.1172/JCI112688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. G., Wong W. W., Schur P. H., Fearon D. T. Mode of inheritance of decreased C3b receptors on erythrocytes of patients with systemic lupus erythematosus. N Engl J Med. 1982 Oct 14;307(16):981–986. doi: 10.1056/NEJM198210143071604. [DOI] [PubMed] [Google Scholar]
- Wong W. W., Cahill J. M., Rosen M. D., Kennedy C. A., Bonaccio E. T., Morris M. J., Wilson J. G., Klickstein L. B., Fearon D. T. Structure of the human CR1 gene. Molecular basis of the structural and quantitative polymorphisms and identification of a new CR1-like allele. J Exp Med. 1989 Mar 1;169(3):847–863. doi: 10.1084/jem.169.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. W., Wilson J. G., Fearon D. T. Genetic regulation of a structural polymorphism of human C3b receptor. J Clin Invest. 1983 Aug;72(2):685–693. doi: 10.1172/JCI111018. [DOI] [PMC free article] [PubMed] [Google Scholar]



