Abstract
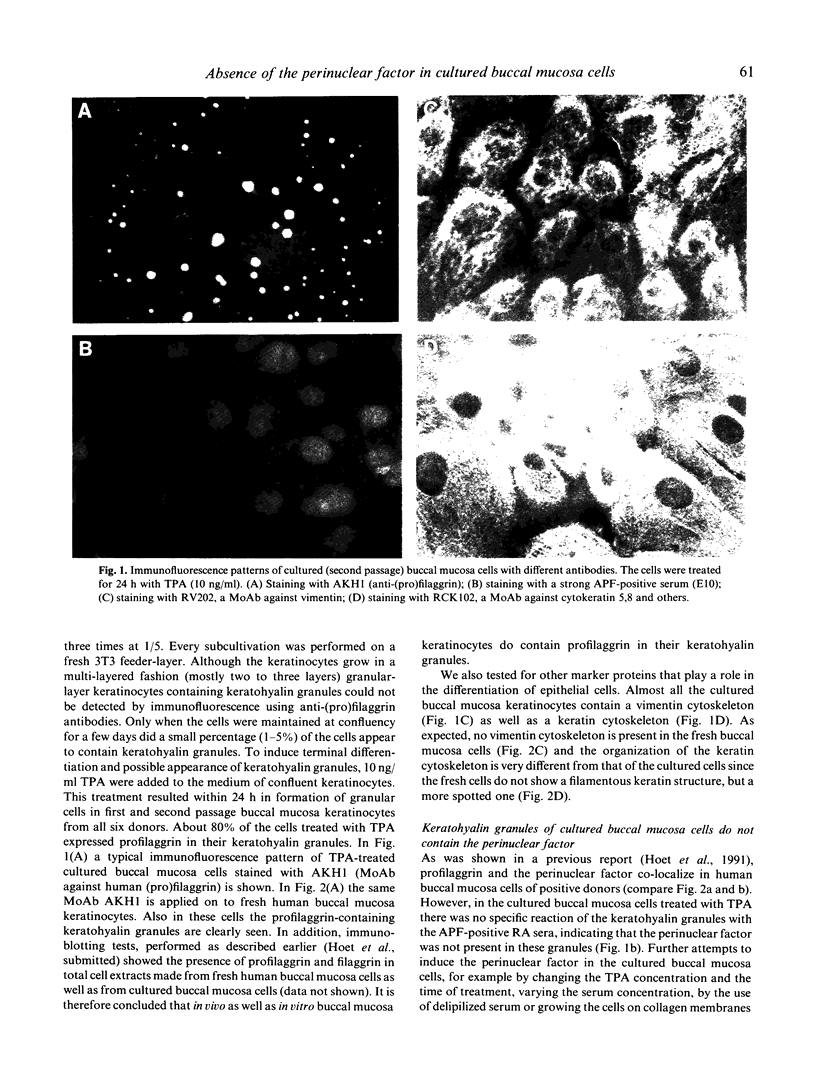
Rheumatoid arthritis patients have antibodies in their serum directed against the perinuclear factor, a protein component present in keratohyalin granules in the cytoplasm of human buccal mucosa cells. The anti-perinuclear factor (APF) can only be detected by an indirect immunofluorescence test performed on fresh buccal mucosa cells from 'selected donors'. To obtain a more reliable antigen source and to gain more insight into the origin and nature of the perinuclear factor we attempted to culture perinuclear factor-containing buccal mucosa cells. Here we describe the successful culturing of such cells, which, however, did not contain keratohyalin granules nor the perinuclear factor. By adding the phorbol ester 12-o-tetradecanoylphorbol-13-acetate (TPA) we were able to induce keratohyalin granules in both cultured primary buccal mucosa cells and a squamous carcinoma cell line of the cheek (SqCC/Y1). These induced keratohyalin granules do contain the protein profilaggrin, which in vivo, in fresh buccal mucosa cells, co-localizes with the perinuclear factor. However, we were not able to demonstrate the presence of the perinuclear factor, not even after induction of terminal differentiation of the cultured cells nor after Epstein-Barr virus infection. Our results suggest that the perinuclear factor, in contrast to profilaggrin, is not an integral component of buccal mucosa cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allday M. J., Crawford D. H. Role of epithelium in EBV persistence and pathogenesis of B-cell tumours. Lancet. 1988 Apr 16;1(8590):855–857. doi: 10.1016/s0140-6736(88)91604-2. [DOI] [PubMed] [Google Scholar]
- Arenholt-Bindslev D., Jepsen A., MacCallum D. K., Lillie J. H. The growth and structure of human oral keratinocytes in culture. J Invest Dermatol. 1987 Mar;88(3):314–319. doi: 10.1111/1523-1747.ep12466191. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Gown A. M., Fleckman P., Kimball J. R., Resing K. A. Characterization of two monoclonal antibodies to human epidermal keratohyalin: reactivity with filaggrin and related proteins. J Invest Dermatol. 1987 Mar;88(3):306–313. doi: 10.1111/1523-1747.ep12466185. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Kimball J. R., Hoff M., Sun T. T. Expression of epidermal keratins and filaggrin during human fetal skin development. J Cell Biol. 1985 Oct;101(4):1257–1269. doi: 10.1083/jcb.101.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. Epstein-Barr virus and human autoimmune diseases: possibilities and pitfalls. J Virol Methods. 1988 Sep;21(1-4):19–27. doi: 10.1016/0166-0934(88)90049-3. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P., Stanley J. R., Schmidt J., Gullino M., Yuspa S. H. The tumor promoter, 12-O-tetradecanoylphorbol-13-acetate accelerates keratinocyte differentiation and stimulates growth of an unidentified cell type in cultured human epidermis. Exp Cell Res. 1982 Jan;137(1):155–167. doi: 10.1016/0014-4827(82)90017-9. [DOI] [PubMed] [Google Scholar]
- Holbrook K. A. Biologic structure and function: perspectives on morphologic approaches to the study of the granular layer keratinocyte. J Invest Dermatol. 1989 Apr;92(4 Suppl):84S–104S. doi: 10.1111/1523-1747.ep13075079. [DOI] [PubMed] [Google Scholar]
- Janssens X., Veys E. M., Verbruggen G., Declercq L. The diagnostic significance of the antiperinuclear factor for rheumatoid arthritis. J Rheumatol. 1988 Sep;15(9):1346–1350. [PubMed] [Google Scholar]
- Jessen H., Peters P. D., Hall T. A. Sulphur in different types of keratohyalin granules: a quantitative assay by X-ray microanalysis. J Cell Sci. 1974 Jul;15(2):359–377. doi: 10.1242/jcs.15.2.359. [DOI] [PubMed] [Google Scholar]
- Kataaha P. K., Mortazavi-Milani S. M., Russell G., Holborow E. J. Anti-intermediate filament antibodies, antikeratin antibody, and antiperinuclear factor in rheumatoid arthritis and infectious mononucleosis. Ann Rheum Dis. 1985 Jul;44(7):446–449. doi: 10.1136/ard.44.7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Bernstein I. A. Exposure to 12-O-tetradecanoylphorbol-13-acetate (TPA) induces the synthesis of histidine-rich protein (filaggrin) in monolayer cultures of rat keratinocytes. J Invest Dermatol. 1987 May;88(5):624–629. doi: 10.1111/1523-1747.ep12470230. [DOI] [PubMed] [Google Scholar]
- NIENHUIS R. L., MANDEMA E. A NEW SERUM FACTOR IN PATIENTS WITH RHEUMATOID ARTHRITIS; THE ANTIPERINUCLEAR FACTOR. Ann Rheum Dis. 1964 Jul;23:302–305. doi: 10.1136/ard.23.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J., Rhodes G., Roudier J., Vaughan J. H. Altered immune response to glycine-rich sequences of Epstein-Barr nuclear antigen-1 in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 1990 Jul;33(7):993–1000. doi: 10.1002/art.1780330711. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Schaart G., Moesker O., Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987 May;170(1):235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rothblat G. H., Arbogast L. Y., Ouellette L., Howard B. V. Preparation of delipidized serum protein for use in cell culture systems. In Vitro. 1976 Aug;12(8):554–557. doi: 10.1007/BF02797438. [DOI] [PubMed] [Google Scholar]
- Smit J. W., Sondag-Tschroots I. R., Aaij C., Feltkamp T. E., Feltkamp-Vroom T. M. The antiperinuclear factor: II. A light microscopical and immunofluorescence study on the antigenic substrate. Ann Rheum Dis. 1980 Aug;39(4):381–386. doi: 10.1136/ard.39.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondag-Tschroots I. R., Aaij C., Smit J. W., Feltkamp T. E. The antiperinuclear factor. 1. The diagnostic significance of the antiperinuclear factor for rheumatoid arthritis. Ann Rheum Dis. 1979 Jun;38(3):248–251. doi: 10.1136/ard.38.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoerker J., Yajima Y., Glaser R. The interaction of non-transforming Epstein-Barr virus (EBV) with cell-associated EBV DNA in superinfected lymphoblastoid cell lines. Int J Cancer. 1982 Oct 15;30(4):385–392. doi: 10.1002/ijc.2910300402. [DOI] [PubMed] [Google Scholar]
- Tomei L. D., Noyes I., Blocker D., Holliday J., Glaser R. Phorbol ester and Epstein-Barr virus dependent transformation of normal primary human skin epithelial cells. Nature. 1987 Sep 3;329(6134):73–75. doi: 10.1038/329073a0. [DOI] [PubMed] [Google Scholar]
- Venables P. J., Roffe L. M., Erhardt C. C., Maini R. N., Edwards J. M., Porter A. D. Titers of antibodies to RANA in rheumatoid arthritis and normal sera. Relationship to Epstein-Barr virus infection. Arthritis Rheum. 1981 Dec;24(12):1459–1468. doi: 10.1002/art.1780241201. [DOI] [PubMed] [Google Scholar]
- Violette S. M., King I., Sartorelli A. C. Antagonistic effects of retinoic acid and hydrocortisone on terminal differentiation of human squamous carcinoma cells. J Invest Dermatol. 1989 Jul;93(1):165–168. doi: 10.1111/1523-1747.ep12277393. [DOI] [PubMed] [Google Scholar]
- Vivino F. B., Maul G. G. Histologic and electron microscopic characterization of the antiperinuclear factor antigen. Arthritis Rheum. 1990 Jul;33(7):960–969. doi: 10.1002/art.1780330707. [DOI] [PubMed] [Google Scholar]
- Watt F. M. Keratinocyte cultures: an experimental model for studying how proliferation and terminal differentiation are co-ordinated in the epidermis. J Cell Sci. 1988 Aug;90(Pt 4):525–529. doi: 10.1242/jcs.90.4.525. [DOI] [PubMed] [Google Scholar]
- Westgeest A. A., Boerbooms A. M., Jongmans M., Vandenbroucke J. P., Vierwinden G., van de Putte L. B. Antiperinuclear factor: indicator of more severe disease in seronegative rheumatoid arthritis. J Rheumatol. 1987 Oct;14(5):893–897. [PubMed] [Google Scholar]
- Westgeest A. A., van Loon A. M., van der Logt J. T., van de Putte L. B., Boerbooms A. M. Antiperinuclear factor, a rheumatoid arthritis specific autoantibody: its relation to Epstein-Barr virus. J Rheumatol. 1989 May;16(5):626–630. [PubMed] [Google Scholar]



