Abstract
Mycobacterium leprae antigens could be detected in the cerebrospinal fluid (CSF) of patients with leprosy, using a monoclonal-antibody-based sandwich immunoradiometric assay (SIRMA). Antigens of 12 kD, 35 kD and 30-40 kD were detected using ML06, ML04, and ML34 monoclonal antibodies, respectively. The 30-40-kD polysaccharide antigen, although present in larger amounts in M. leprae than the 12-kD and 35-kD protein antigens, was found in the CSF of comparatively fewer subjects. The antigen capture assay has been found sensitive to the level of nanograms. Avidin-biotin-based immunoblotting using pooled leprosy sera detected a larger number of antigens than using anti-M. leprae antisera raised in rabbits. The immunoblotting of CSF samples revealed about three antigens in the region of 100-160 kD and three more in the region of 45-60 kD as probed by leprosy sera. This study has for the first time revealed the presence of M. leprae antigens in the CSF of leprosy patients and the probable involvement of the central nervous system in leprosy.
Full text
PDF

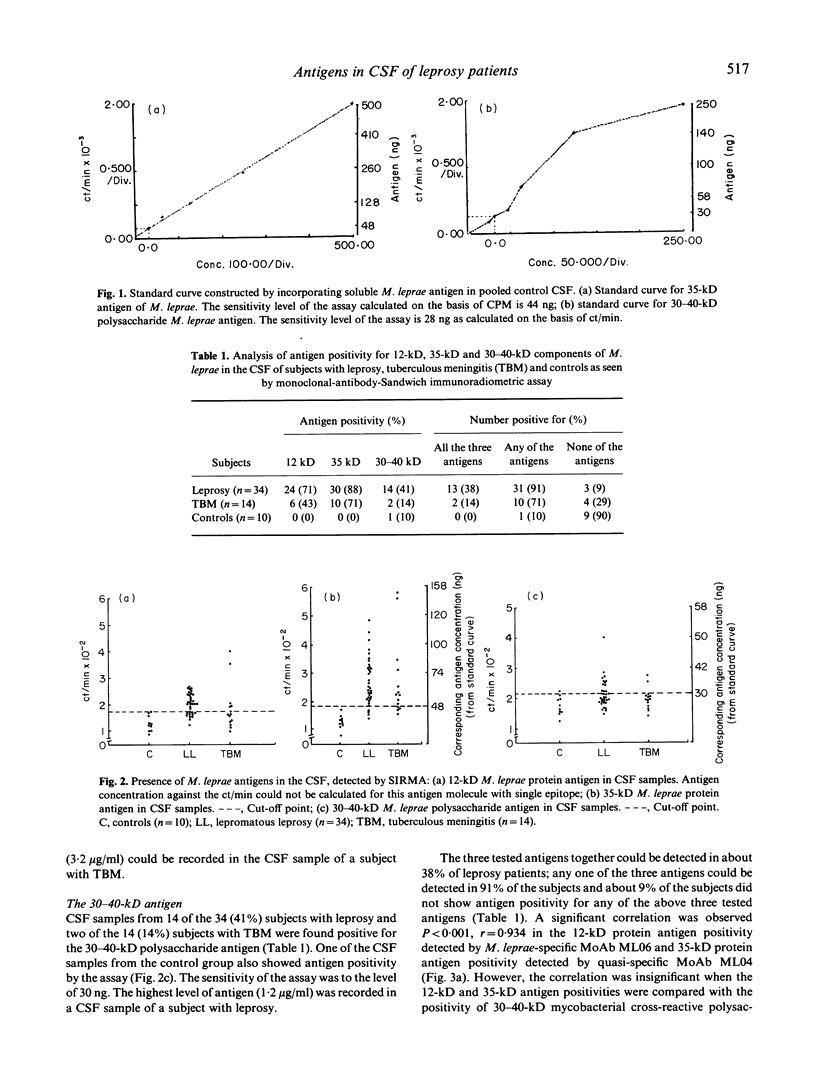
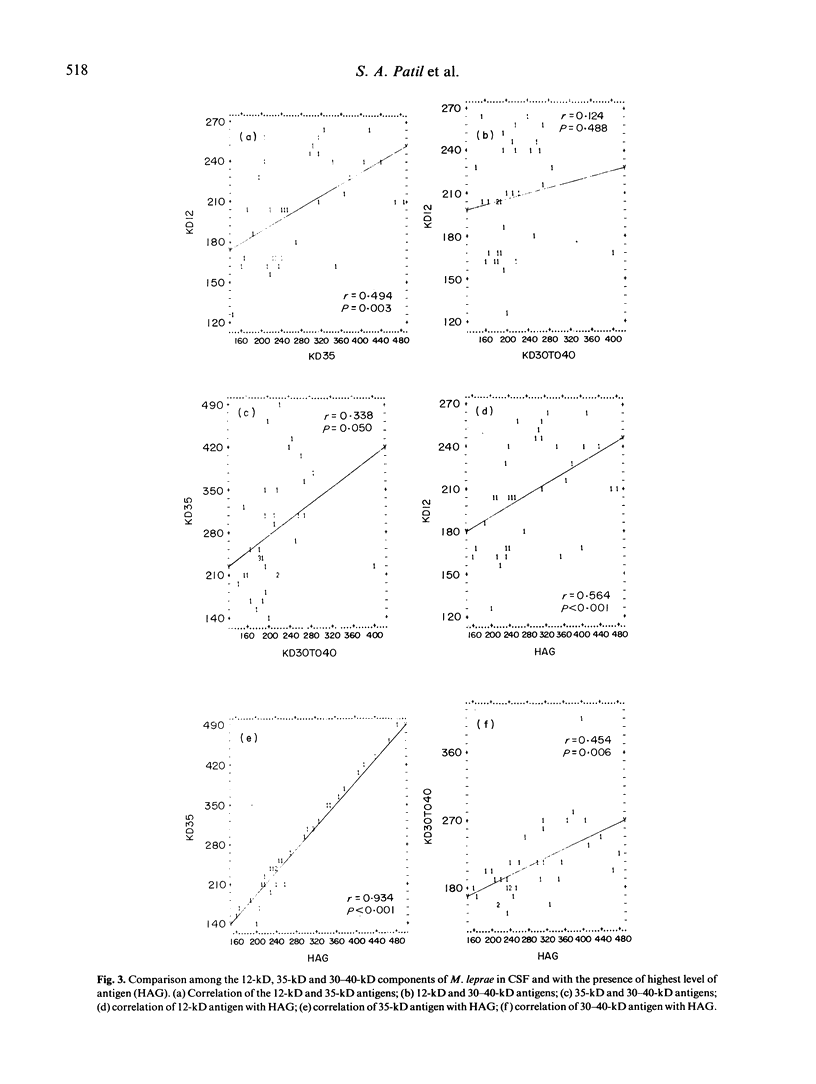
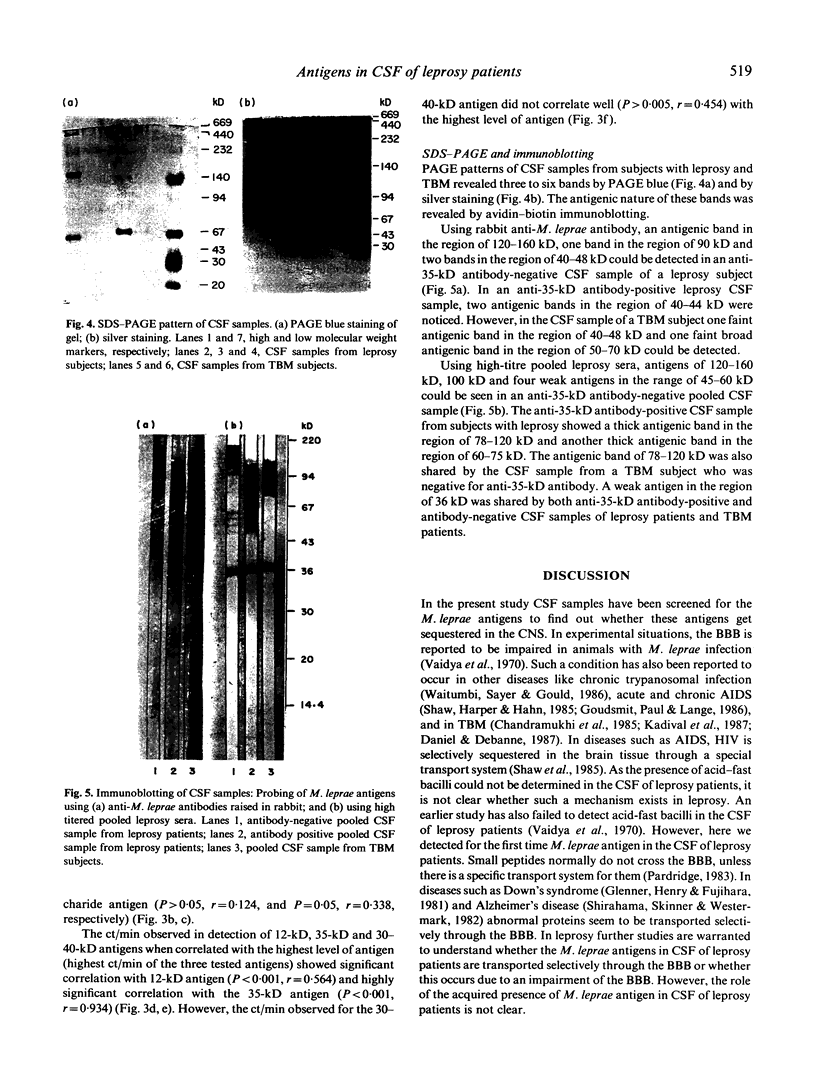


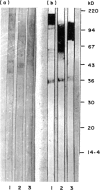
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boddingius J. The occurrence of Mycobacterium leprae within axons of peripheral nerves. Acta Neuropathol. 1974 Mar 26;27(3):257–270. doi: 10.1007/BF00687635. [DOI] [PubMed] [Google Scholar]
- Bothamley G., Swanson Beck J., Agusni I., Ilias M. I., Kardjito T., Grange J. M., Ivanyi J. Antibodies to Mycobacterium tuberculosis in leprosy. Lancet. 1987 May 9;1(8541):1098–1098. doi: 10.1016/s0140-6736(87)90532-0. [DOI] [PubMed] [Google Scholar]
- Chandramuki A., Allen P. R., Keen M., Ivanyi J. Detection of mycobacterial antigen and antibodies in the cerebrospinal fluid of patients with tuberculous meningitis. J Med Microbiol. 1985 Oct;20(2):239–247. doi: 10.1099/00222615-20-2-239. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Debanne S. M. The serodiagnosis of tuberculosis and other mycobacterial diseases by enzyme-linked immunosorbent assay. Am Rev Respir Dis. 1987 May;135(5):1137–1151. doi: 10.1164/arrd.1987.135.5.1137. [DOI] [PubMed] [Google Scholar]
- Drutz D. J., Chen T. S., Lu W. H. The continuous bacteremia of lepromatous leprosy. N Engl J Med. 1972 Jul 27;287(4):159–164. doi: 10.1056/NEJM197207272870402. [DOI] [PubMed] [Google Scholar]
- Fine P. Leprosy and tuberculosis--an epidemiological comparison. Tubercle. 1984 Jun;65(2):137–153. doi: 10.1016/0041-3879(84)90067-9. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fryden A., Link H., Norrby E. Cerebrospinal fluid and serum immunoglobulins and antibody titers in mumps meningitis and aseptic meningitis of other etiology. Infect Immun. 1978 Sep;21(3):852–861. doi: 10.1128/iai.21.3.852-861.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Henry J. H., Fujihara S. Congophilic angiopathy in the pathogenesis of Alzheimer's degeneration. Ann Pathol. 1981;1(2):120–129. [PubMed] [Google Scholar]
- Goudsmit J., de Wolf F., Paul D. A., Epstein L. G., Lange J. M., Krone W. J., Speelman H., Wolters E. C., Van der Noordaa J., Oleske J. M. Expression of human immunodeficiency virus antigen (HIV-Ag) in serum and cerebrospinal fluid during acute and chronic infection. Lancet. 1986 Jul 26;2(8500):177–180. doi: 10.1016/s0140-6736(86)92485-2. [DOI] [PubMed] [Google Scholar]
- Griffin D. E., Giffels J. Study of protein characteristics that influence entry into the cerebrospinal fluid of normal mice and mice with encephalitis. J Clin Invest. 1982 Aug;70(2):289–295. doi: 10.1172/JCI110616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Harboe M., Ivanyi J. Analysis of monoclonal antibodies to Mycobacterium leprae by crossed immunoelectrophoresis. Scand J Immunol. 1987 Feb;25(2):133–138. doi: 10.1111/j.1365-3083.1987.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Hewitt J., Coates A. R., Mitchison D. A., Ivanyi J. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J Immunol Methods. 1982 Dec 17;55(2):205–211. doi: 10.1016/0022-1759(82)90032-1. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Sinha S., Aston R., Cussell D., Keen M., Sengupta U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin Exp Immunol. 1983 Jun;52(3):528–536. [PMC free article] [PubMed] [Google Scholar]
- Kadival G. V., Samuel A. M., Mazarelo T. B., Chaparas S. D. Radioimmunoassay for detecting Mycobacterium tuberculosis antigen in cerebrospinal fluids of patients with tuberculous meningitis. J Infect Dis. 1987 Apr;155(4):608–611. doi: 10.1093/infdis/155.4.608. [DOI] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Hengstler B., Zak O. Cerebrospinal fluid protein profile in experimental pneumococcal meningitis and its alteration by ampicillin and anti-inflammatory agents. J Infect Dis. 1989 Jan;159(1):26–34. doi: 10.1093/infdis/159.1.26. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskin O. L., Griffin D. E. Changes in cerebrospinal fluid cells, IgG and IgA during herpes simplex virus encephalitis in rabbits. J Neuroimmunol. 1987 Apr;14(3):283–292. doi: 10.1016/0165-5728(87)90015-4. [DOI] [PubMed] [Google Scholar]
- Mshana R. N., Humber D. P., Harboe M., Belehu A. Demonstration of mycobacterial antigens in nerve biopsies from leprosy patients using peroxidase-antiperoxidase immunoenzyme technique. Clin Immunol Immunopathol. 1983 Dec;29(3):359–368. doi: 10.1016/0090-1229(83)90039-9. [DOI] [PubMed] [Google Scholar]
- Mwatha J., Moreno C., Sengupta U., Sinha S., Ivanyi J. A comparative evaluation of serological assays for lepromatous leprosy. Lepr Rev. 1988 Sep;59(3):195–199. doi: 10.5935/0305-7518.19880024. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Neuropeptides and the blood-brain barrier. Annu Rev Physiol. 1983;45:73–82. doi: 10.1146/annurev.ph.45.030183.000445. [DOI] [PubMed] [Google Scholar]
- Patil S. A., Girdhar B. K., Singh K. P., Sengupta U. Detection of Mycobacterium leprae antigens in the sera of leprosy patients by sandwich immunoradiometric assay using monoclonal antibodies. J Clin Microbiol. 1990 Dec;28(12):2792–2796. doi: 10.1128/jcm.28.12.2792-2796.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliarello V. J., Long W. J., Scheld W. M. Morphologic alterations of the blood-brain barrier with experimental meningitis in the rat. Temporal sequence and role of encapsulation. J Clin Invest. 1986 Apr;77(4):1084–1095. doi: 10.1172/JCI112407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Shirahama T., Skinner M., Westermark P., Rubinow A., Cohen A. S., Brun A., Kemper T. L. Senile cerebral amyloid. Prealbumin as a common constituent in the neuritic plaque, in the neurofibrillary tangle, and in the microangiopathic lesion. Am J Pathol. 1982 Apr;107(1):41–50. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya M. C., Palmer E., Weddell G., Rees R. J. A note on the presence of Mycobacterium leprae in the central nervous system of a mouse with lepromatous leprosy. J Med Microbiol. 1970 Feb;3(1):194–196. doi: 10.1099/00222615-3-1-194. [DOI] [PubMed] [Google Scholar]
- Waitumbi J. N., Sayer P. D., Gould S. S. Evidence of blood cerebrospinal fluid barrier permeability impairment in chronic Trypanosoma rhodesiense infection in a vervet monkey. Trans R Soc Trop Med Hyg. 1986;80(5):848–848. doi: 10.1016/0035-9203(86)90402-5. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]