Abstract
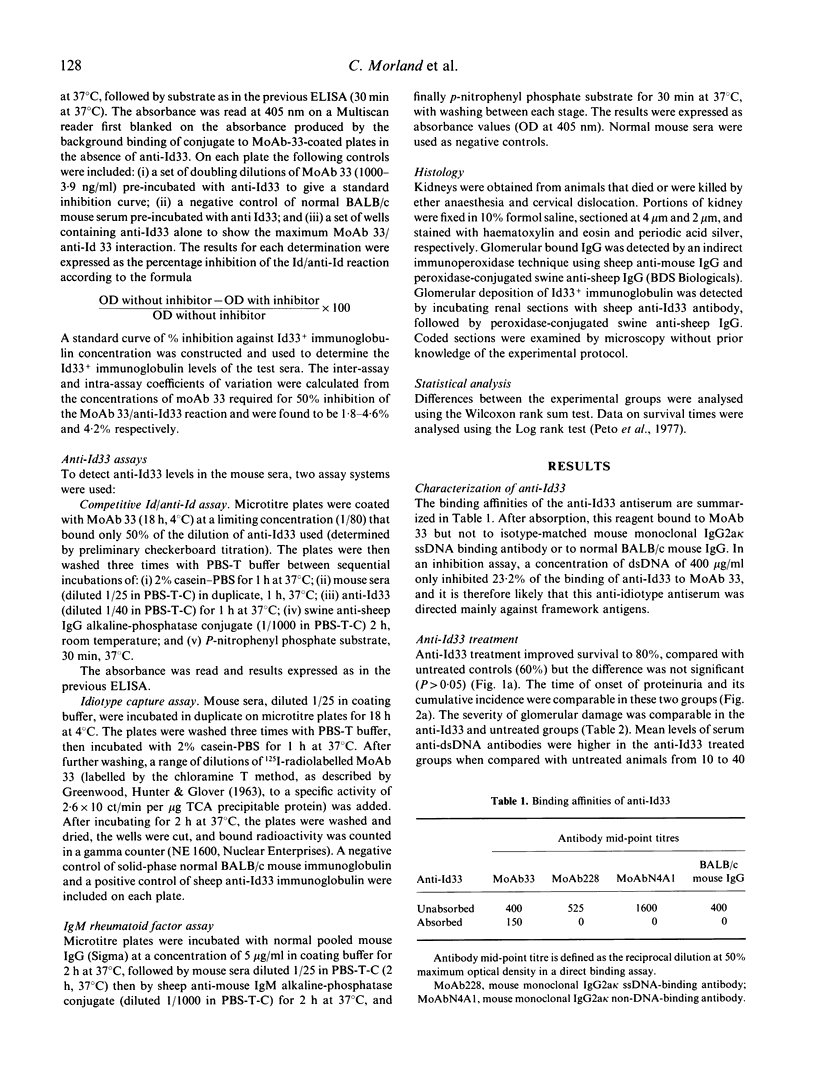
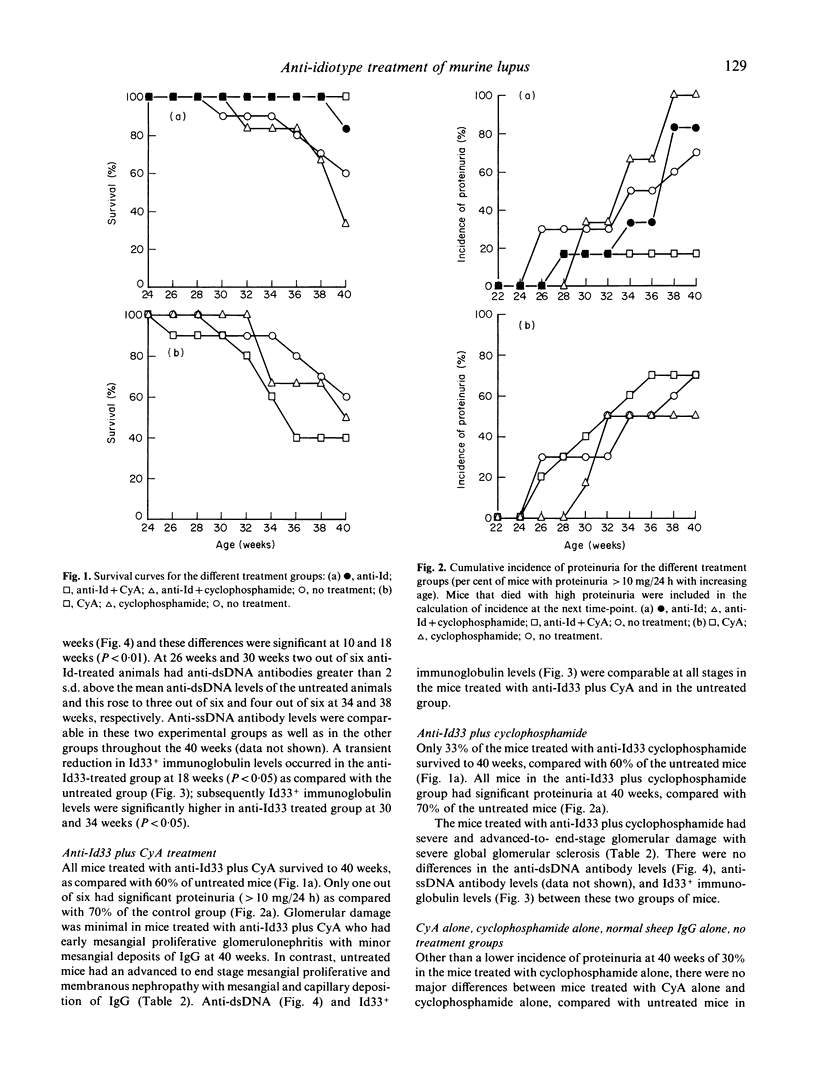
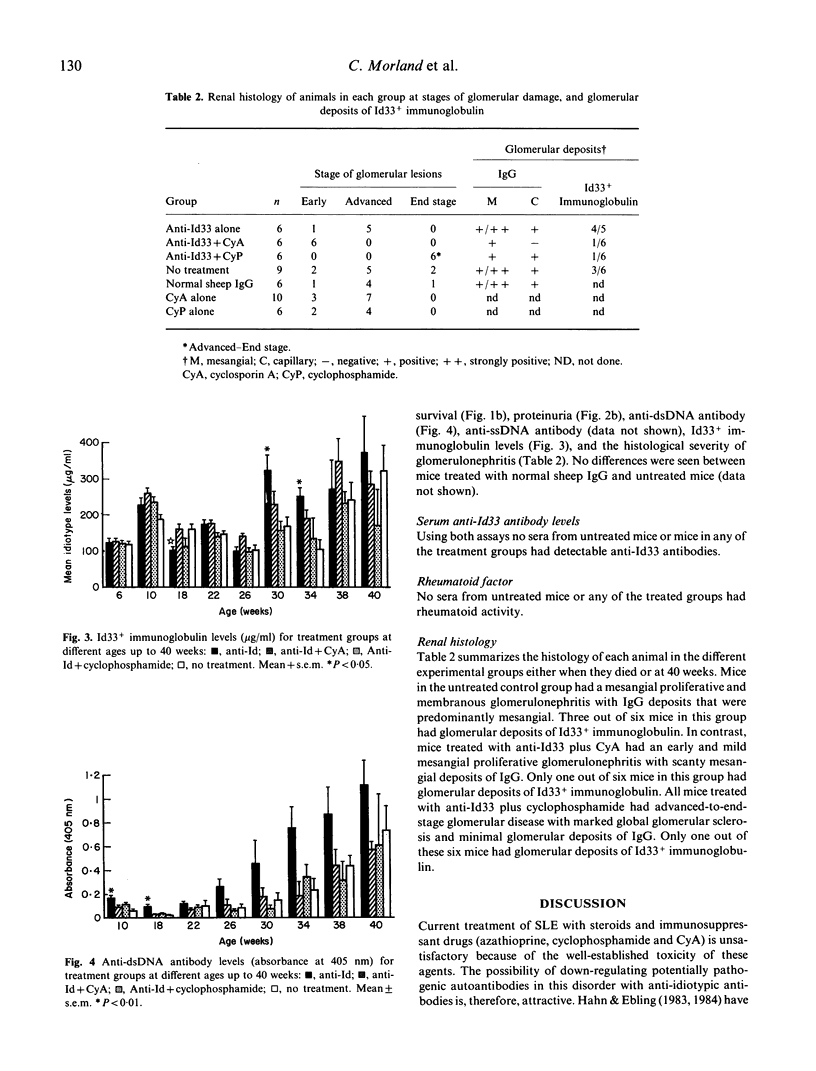
The effect of the administration of a xenogeneic anti-idiotype antibody (anti-Id33) to a cross-reactive idiotype (Id33) present on anti-dsDNA antibody was examined in 6-week-old (NZB/NZW) F1 (BWF1) female mice. The administration of anti-Id33 led to a transient reduction in immunoglobulins expressing Id33, followed by a rise at 30 and 34 weeks that was significantly higher than in untreated mice (P less than 0.05). Likewise, anti-dsDNA antibody levels were significantly higher at 10 and 18 weeks than in untreated mice (P less than 0.01). No differences were seen in survival to 40 weeks, proteinuria or the severity of glomerulonephritis. Concurrent administration of cyclosporin A (CyA) with anti-Id33 markedly ameliorated glomerular injury and proteinuria and improved survival. By contrast, glomerular injury, proteinuria and survival were worse in mice treated with cyclophosphamide plus anti-Id33, compared with untreated mice. Neither CyA nor cyclophosphamide treatment, when given with anti-Id33 altered serum levels of anti-dsDNA, anti-ssDNA or Id33+ immunoglobin, compared with untreated mice. The different effects of CyA and cyclophosphamide on T lymphocytes and their discrepant effects on glomerular injury when given with anti-Id33 in this model lead us to postulate a role for T lymphocytes in the glomerular injury of BWF1 lupus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Collins A. B., Schneeberger E. E., McCluskey R. T. A cell-mediated reaction against glomerular-bound immune complexes. J Exp Med. 1979 Dec 1;150(6):1410–1420. doi: 10.1084/jem.150.6.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce N. W., Tipping P. G., Holdsworth S. R. Lymphokine (MIF) production by glomerular T-lymphocytes in experimental glomerulonephritis. Kidney Int. 1986 Nov;30(5):673–677. doi: 10.1038/ki.1986.239. [DOI] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Bergui L., Tesio L., Ziano R., Camussi G. HLA-Dr+ T cells of the Leu 3 (helper) type infiltrate the kidneys of patients with systemic lupus erythematosus. Clin Exp Immunol. 1985 Jan;59(1):185–189. [PMC free article] [PubMed] [Google Scholar]
- Ebling F., Hahn B. H. Restricted subpopulations of DNA antibodies in kidneys of mice with systemic lupus. Comparison of antibodies in serum and renal eluates. Arthritis Rheum. 1980 Apr;23(4):392–403. doi: 10.1002/art.1780230402. [DOI] [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. I. Influence of the dose and of the effector functions of anti-idiotypic antibody on the production of an idiotype. Eur J Immunol. 1974 Apr;4(4):296–302. doi: 10.1002/eji.1830040413. [DOI] [PubMed] [Google Scholar]
- Epstein A., Greenberg M., Diamond B., Grayzel A. I. Suppression of anti-DNA antibody synthesis in vitro by a cross-reactive antiidiotypic antibody. J Clin Invest. 1987 Mar;79(3):997–1000. doi: 10.1172/JCI112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Ebling F. M. Suppression of NZB/NZW murine nephritis by administration of a syngeneic monoclonal antibody to DNA. Possible role of anti-idiotypic antibodies. J Clin Invest. 1983 Jun;71(6):1728–1736. doi: 10.1172/JCI110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Ebling F. M. Suppression of murine lupus nephritis by administration of an anti-idiotypic antibody to anti-DNA. J Immunol. 1984 Jan;132(1):187–190. [PubMed] [Google Scholar]
- Hart D. A., Wang A. L., Pawlak L. L., Nisonoff A. Suppression of idiotypic specificities in adult mice by administration of antiidiotypic antibody. J Exp Med. 1972 Jun 1;135(6):1293–1300. doi: 10.1084/jem.135.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A. D., Colombani P. M. Cyclosporin. Mechanism of action: in vitro studies. Prog Allergy. 1986;38:198–221. [PubMed] [Google Scholar]
- Holdsworth S. R., Allen D. E., Thomson N. M., Glasgow E. F., Atkins R. C. Histochemistry of glomerular cells in animal models of crescentic glomerulonephritis. Pathology. 1980 Jul;12(3):339–346. doi: 10.3109/00313028009077095. [DOI] [PubMed] [Google Scholar]
- Hooke D. H., Hancock W. W., Gee D. C., Kraft N., Atkins R. C. Monoclonal antibody analysis of glomerular hypercellularity in human glomerulonephritis. Clin Nephrol. 1984 Oct;22(4):163–168. [PubMed] [Google Scholar]
- Isenberg D. A., Collins C. Detection of cross-reactive anti-DNA antibody idiotypes on renal tissue-bound immunoglobulins from lupus patients. J Clin Invest. 1985 Jul;76(1):287–294. doi: 10.1172/JCI111959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg D. A., Staines N. A. DNA antibody idiotypes. An analysis of their role in health and disease. J Autoimmun. 1990 Aug;3(4):339–356. doi: 10.1016/s0896-8411(05)80002-2. [DOI] [PubMed] [Google Scholar]
- Jacob L., Tron F. Induction of anti-DNA autoanti-idiotypic antibodies in (NZB X NZW)F1 mice: possible role for specific immune suppression. Clin Exp Immunol. 1984 Nov;58(2):293–299. [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Jørgensen T., Hannestad K. H--2-linked genes control immune response to V-domains of myeloma protein 315. Nature. 1980 Nov 27;288(5789):396–397. doi: 10.1038/288396a0. [DOI] [PubMed] [Google Scholar]
- Lake R. A., Staines N. A. A monoclonal DNA-binding autoantibody causes a deterioration in renal function in MRL mice with lupus disease. Clin Exp Immunol. 1988 Jul;73(1):103–110. [PMC free article] [PubMed] [Google Scholar]
- Mahana W., Guilbert B., Avrameas S. Suppression of anti-DNA antibody production in MRL mice by treatment with anti-idiotypic antibodies. Clin Exp Immunol. 1987 Dec;70(3):538–545. [PMC free article] [PubMed] [Google Scholar]
- Marion T. N., Lawton A. R., 3rd, Kearney J. F., Briles D. E. Anti-DNA autoantibodies in (NZB X NZW)F1 mice are clonally heterogeneous, but the majority share a common idiotype. J Immunol. 1982 Feb;128(2):668–674. [PubMed] [Google Scholar]
- Mendlovic S., Brocke S., Shoenfeld Y., Ben-Bassat M., Meshorer A., Bakimer R., Mozes E. Induction of a systemic lupus erythematosus-like disease in mice by a common human anti-DNA idiotype. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2260–2264. doi: 10.1073/pnas.85.7.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlovic S., Shoenfeld Y., Bakimer R., Segal R., Dayan M., Mozes E. In vitro T-cell functions specific to an anti-DNA idiotype and serological markers in patients with systemic lupus erythematosus (SLE). J Clin Immunol. 1988 May;8(3):178–187. doi: 10.1007/BF00917564. [DOI] [PubMed] [Google Scholar]
- Morgan A., Buchanan R. R., Lew A. M., Olsen I., Staines N. A. Five groups of antigenic determinants on DNA identified by monoclonal antibodies from (NZB X NZW)F1 and MRL/Mp-lpr/lpr mice. Immunology. 1985 May;55(1):75–83. [PMC free article] [PubMed] [Google Scholar]
- Morgan A., Isenberg D. A., Naparstek Y., Rauch J., Duggan D., Khiroya R., Staines N. A., Schattner A. Shared idiotypes are expressed on mouse and human anti-DNA autoantibodies. Immunology. 1985 Nov;56(3):393–399. [PMC free article] [PubMed] [Google Scholar]
- Nolasco F. E., Cameron J. S., Hartley B., Coelho A., Hildreth G., Reuben R. Intraglomerular T cells and monocytes in nephritis: study with monoclonal antibodies. Kidney Int. 1987 May;31(5):1160–1166. doi: 10.1038/ki.1987.123. [DOI] [PubMed] [Google Scholar]
- Pesce A. J. Methods used for the analysis of proteins in the urine. Nephron. 1974;13(1):93–104. doi: 10.1159/000180371. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch J., Murphy E., Roths J. B., Stollar B. D., Schwartz R. S. A high frequency idiotypic marker of anti-DNA autoantibodies in MRL-Ipr/Ipr mice. J Immunol. 1982 Jul;129(1):236–241. [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A., Rauch J., Madaio M. P., Stollar B. D., Schwartz R. S. Idiotypic cross-reactions of monoclonal human lupus autoantibodies. J Exp Med. 1983 Sep 1;158(3):718–730. doi: 10.1084/jem.158.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon G., Schiffenbauer J., Keiser H. D., Diamond B. Use of monoclonal antibodies to identify shared idiotypes on human antibodies to native DNA from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1983 Feb;80(3):850–854. doi: 10.1073/pnas.80.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D., Rauch J., Stollar B. D., Schwartz R. S. In vivo effects of antibodies against a high frequency idiotype of anti-DNA antibodies in MRL mice. J Immunol. 1984 Mar;132(3):1282–1285. [PubMed] [Google Scholar]
- Tipping P. G., Neale T. J., Holdsworth S. R. T lymphocyte participation in antibody-induced experimental glomerulonephritis. Kidney Int. 1985 Mar;27(3):530–537. doi: 10.1038/ki.1985.43. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Parker D. Effect of cyclophosphamide on immunological control mechanisms. Immunol Rev. 1982;65:99–113. doi: 10.1111/j.1600-065x.1982.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Bitoh S., Torii M., Fujimoto S. Idiotype-specific T lymphocytes. I. Regulation of antibody production by idiotype-specific H-2-restricted T lymphocytes. J Immunol. 1983 Mar;130(3):1038–1042. [PubMed] [Google Scholar]
- Zouali M., Jolivet M., Leclerc C., Ravisse P., Audibert F., Eyquem A., Chedio L. Suppression of murine lupus autoantibodies to DNA by administration of muramyl dipeptide and syngeneic anti-DNA IgG. J Immunol. 1985 Aug;135(2):1091–1096. [PubMed] [Google Scholar]


