Abstract
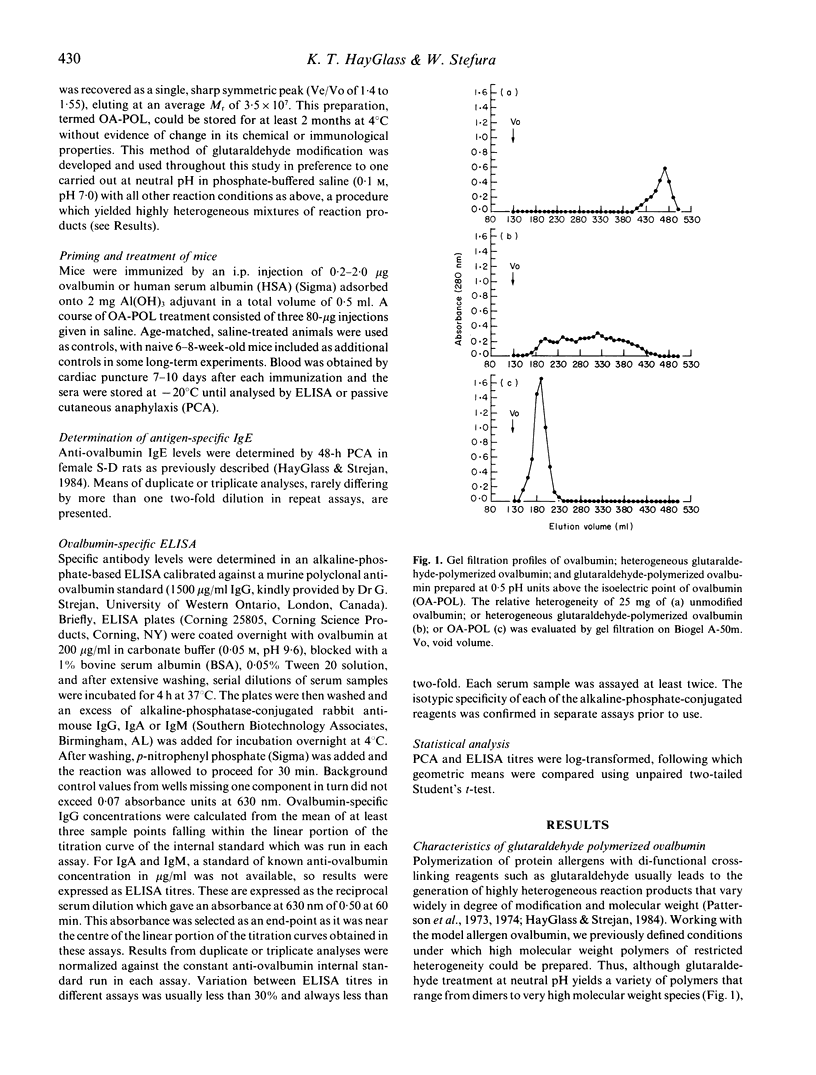
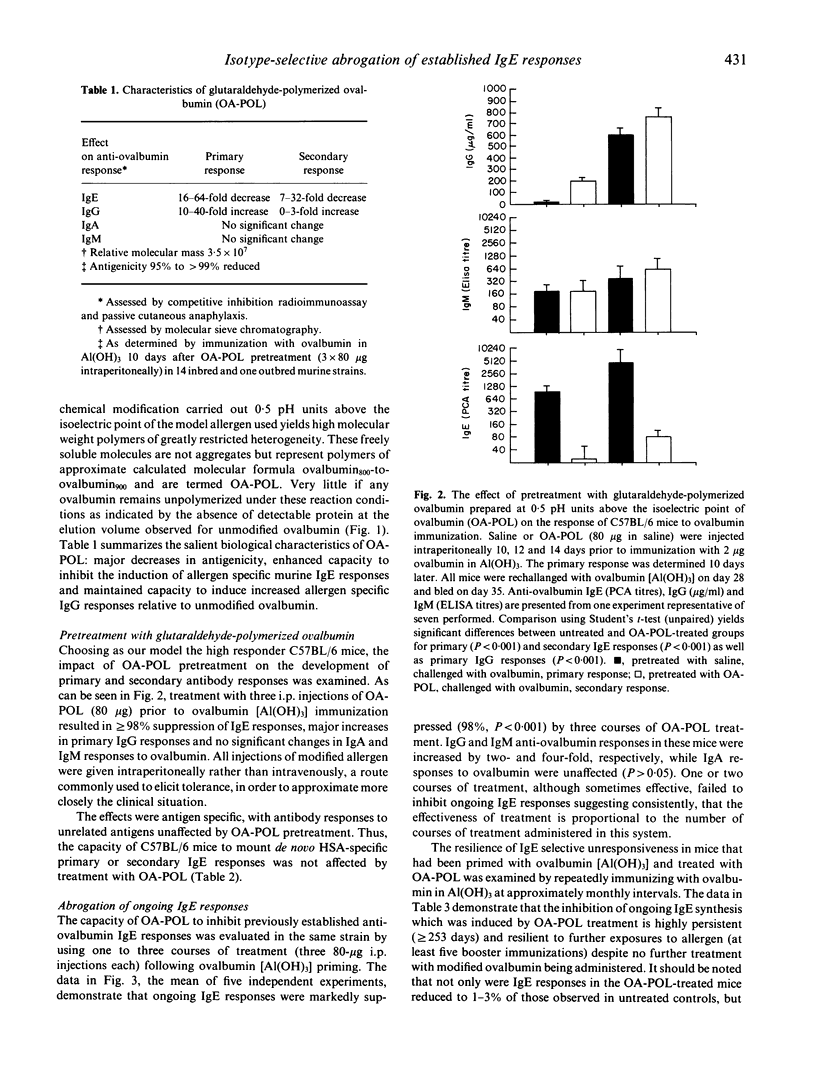
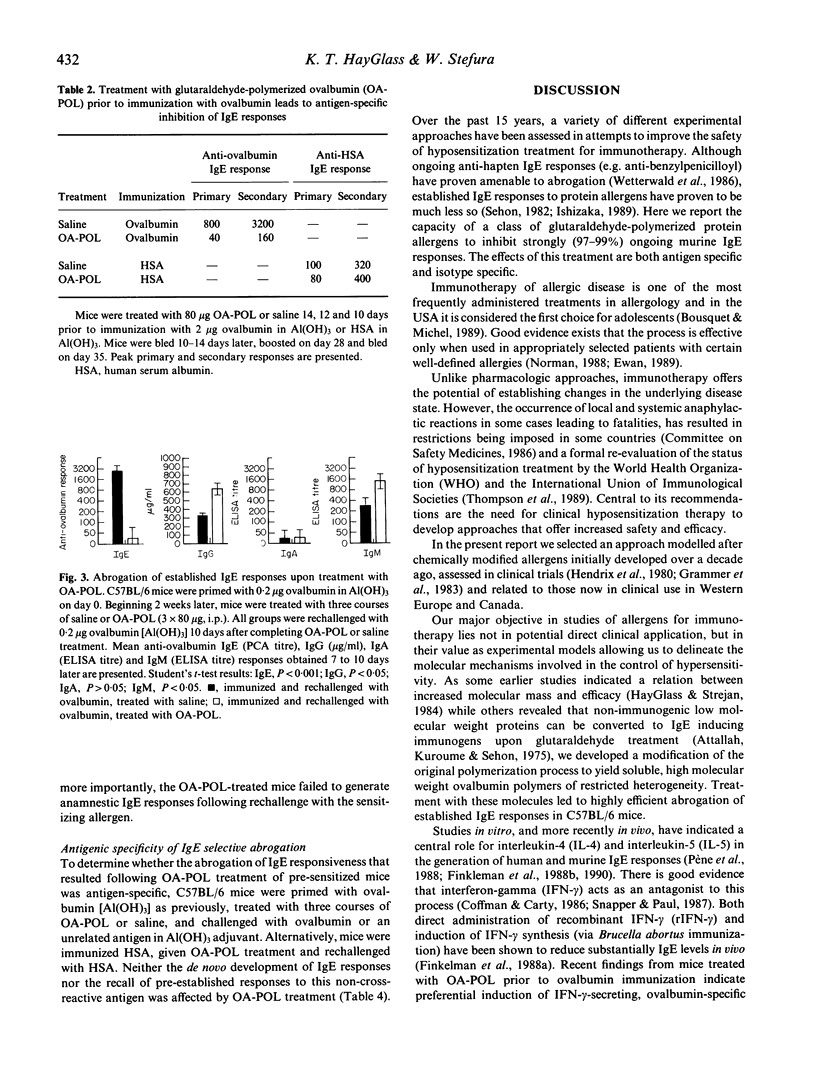
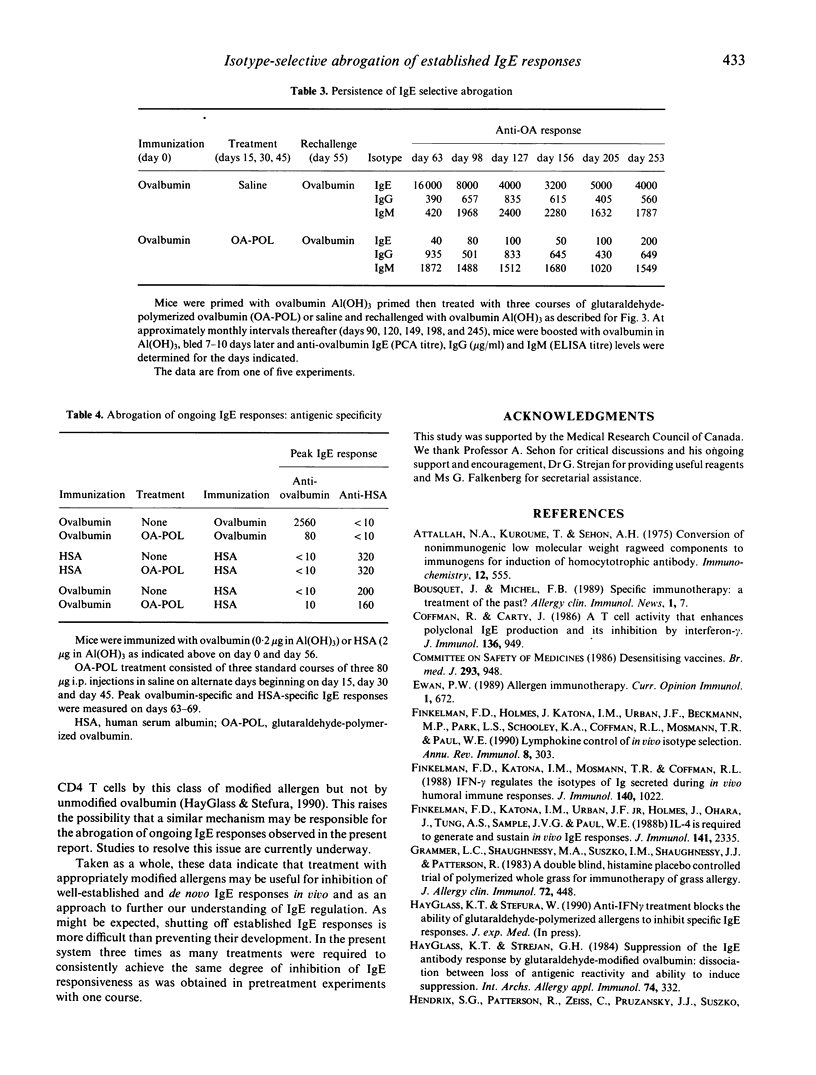
Chemically modified allergens have been extensively studied in an attempt to develop materials of increased efficacy and improved safety for use in the immunotherapy of allergic disease. Most of the strategies that have been developed yield products that strongly inhibit de novo IgE responses but have only marginal impact on ongoing IgE responses. We report the virtual abrogation of pre-established murine anti-ovalbumin IgE responses using a glutaraldehyde-polymerized ovalbumin preparation (OA-POL) of Mr 3.5 x 10(7). Secondary IgE responses are inhibited by 97-99% over a period of at least 8 months following three i.p. courses of OA-POL treatment. Administration of five additional ovalbumin [Al(OH)3] booster immunizations over this period fails to alter this unresponsive state. The inhibition of antigen-specific IgE responses is isotype specific.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attallah N. A., Kuroume T., Sehon A. H. Conversion of nonimmunogenic low molecular weight ragweed components to immunogens for induction of homocytotropic antibody. Immunochemistry. 1975 Jul;12(6-7):555–559. doi: 10.1016/0019-2791(75)90084-1. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Current status of allergen immunotherapy (hyposensitization): memorandum from a WHO/IUIS meeting. Bull World Health Organ. 1989;67(3):263–272. [PMC free article] [PubMed] [Google Scholar]
- Ewan P. W. Allergen immunotherapy. Curr Opin Immunol. 1989 Apr;1(4):672–678. doi: 10.1016/0952-7915(89)90040-x. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Holmes J., Ohara J., Tung A. S., Sample J. V., Paul W. E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988 Oct 1;141(7):2335–2341. [PubMed] [Google Scholar]
- Grammer L. C., Shaughnessy M. A., Suszko I. M., Shaughnessy J. J., Patterson R. A double-blind histamine placebo-controlled trial of polymerized whole grass for immunotherapy of grass allergy. J Allergy Clin Immunol. 1983 Nov;72(5 Pt 1):448–453. doi: 10.1016/0091-6749(83)90580-8. [DOI] [PubMed] [Google Scholar]
- HayGlass K. T., Strejan G. H. Suppression of the IgE antibody response by glutaraldehyde-modified ovalbumin: dissociation between loss of antigenic reactivity and ability to induce suppression. Int Arch Allergy Appl Immunol. 1984;74(4):332–340. doi: 10.1159/000233569. [DOI] [PubMed] [Google Scholar]
- Hendrix S. G., Patterson R., Zeiss C. R., Pruzansky J. J., Suszko I. M., McQueen R. C., Slavin R. G., Miller M. P., Lieberman P. L., Sheffer A. L. A multi-institutional trial of polymerized whole ragweed for immunotherapy of ragweed allergy. J Allergy Clin Immunol. 1980 Dec;66(6):486–494. doi: 10.1016/0091-6749(80)90010-x. [DOI] [PubMed] [Google Scholar]
- Ishizaka K. Regulation of immunoglobin E biosynthesis. Adv Immunol. 1989;47:1–44. [PubMed] [Google Scholar]
- Johansson S. G., Miller A. C., Mullan N., Overell B. G., Tees E. C., Wheeler A. Glutaraldehyde-pollen-tyrosine: clinical and immunological studies. Clin Allergy. 1974 Sep;4(3):255–263. doi: 10.1111/j.1365-2222.1974.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Patterson R., Suszko I. M., McIntire F. C. Polymerized ragweed antigen E. I. Preparation and immunologic studies. J Immunol. 1973 May;110(5):1402–1412. [PubMed] [Google Scholar]
- Patterson R., Suszko I. M. Polymerized ragweed antigen E. 3. Differences in immune response to three molecular weight ranges of monomer and polymer. J Immunol. 1974 May;112(5):1855–1860. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Bonnefoy J. Y., Spits H., Yokota T., Arai N., Arai K., Banchereau J. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6880–6884. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijkers G. T., Mosier D. E. Pneumococcal polysaccharides induce antibody formation by human B lymphocytes in vitro. J Immunol. 1985 Jul;135(1):1–4. [PubMed] [Google Scholar]
- Sehon A. H. Suppression of IgE antibody responses with tolerogenic conjugates of allergens and haptens. Prog Allergy. 1982;32:161–202. [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Wetterwald A., Toffler O., de Weck A. L., Blaser K. Suppression of established IgE antibody responses with isologous anti-idiotypic antibodies in guinea pigs. Mol Immunol. 1986 Mar;23(3):347–356. doi: 10.1016/0161-5890(86)90062-3. [DOI] [PubMed] [Google Scholar]


