Abstract
A low molecular weight nonpeptide compound, KRH-1636, efficiently blocked replication of various T cell line-tropic (X4) HIV type 1 (HIV-1) in MT-4 cells and peripheral blood mononuclear cells through the inhibition of viral entry and membrane fusion via the CXC chemokine receptor (CXCR)4 coreceptor but not via CC chemokine receptor 5. It also inhibited binding of the CXC chemokine, stromal cell-derived factor 1α, to CXCR4 specifically and subsequent signal transduction. KRH-1636 prevented monoclonal antibodies from binding to CXCR4 without down-modulation of the coreceptor. The inhibitory effect against X4 viral replication by KRH-1636 was clearly reproduced in the human peripheral blood lymphocyte/severe combined immunodeficiency mouse system. Furthermore, this compound was absorbed into the blood after intraduodenal administration as judged by anti-HIV-1 activity and liquid chromatography MS in the plasma. Thus, KRH-1636 seems to be a promising agent for the treatment of HIV-1 infection.
To date, highly active antiretroviral therapy for HIV type 1 (HIV-1) has led to a dramatic effect in reduction of the extent of viral load, improvement of CD4+ T cell number status, and often remarkable recovery from disease in infected individuals (1–4). However, due to many factors such as the possible appearance of drug-resistant mutants, side effects, and high cost, there is still a great need for improved therapies.
CXC chemokine receptor (CXCR)4 is a coreceptor for the entry of T cell line-tropic (X4) strains of HIV-1, and CC chemokine receptor (CCR)5 serves as a coreceptor for macrophage-tropic (R5) strains of HIV-1 (5–8). In addition, the CXC chemokine stromal cell-derived factor 1α (SDF-1α) blocks the infection of lymphocytes by X4 HIV-1 isolates (9, 10), and the CC chemokines regulated on activation, normal T cells expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1α, and MIP-1β, which are ligands for CCR5, inhibit the infection of R5 HIV-1 isolates (11). These observations suggest that chemokines or chemokine derivatives or small-molecule chemokine receptor antagonists or agonists may be useful for the treatment of HIV-1 infection. Indeed, several groups of low molecular weight compounds are reported to inhibit HIV-1 infection, including the bicyclam AMD3100 (12–14) and 18-mer peptide T22 (15, 16), which potently block HIV-1 entry and infection through CXCR4. More recently, a nonpeptide small molecular weight compound named TAK-779 was found to be a potent and selective CCR5 antagonist (17). Unfortunately, it is not possible to administer these compounds by the oral route. We believe that oral availability is one of the key issues to be achieved for anti-HIV drugs for the benefit of HIV-1-infected individuals, because HIV-1 infection is chronic and life-long. We therefore focused on the development of orally available chemokine antagonists. This study describes the discovery of a potent CXCR4 antagonist with a small nonpeptide molecule that can be administered orally.
Materials and Methods
Compounds.
The synthesis and purification of KRH-1636, N-{(S)-4-guanidino-1-[(S)-1-naphthalen-1-yl-ethylcarbamoyl]butyl}-4-{[(pyridin-2-yl-methyl)amino]methyl}benzamide (Fig. 1a), its methansulfonated analog, and AMD3100 were carried out at Kureha Chemical Industry (Tokyo). These compounds were dissolved in dimethyl sulfoxide at 10 mM to exclude any antiviral or cytotoxic effect of dimethyl sulfoxide. T22 was kindly supplied from Seikagaku Kogyo (Tokyo).
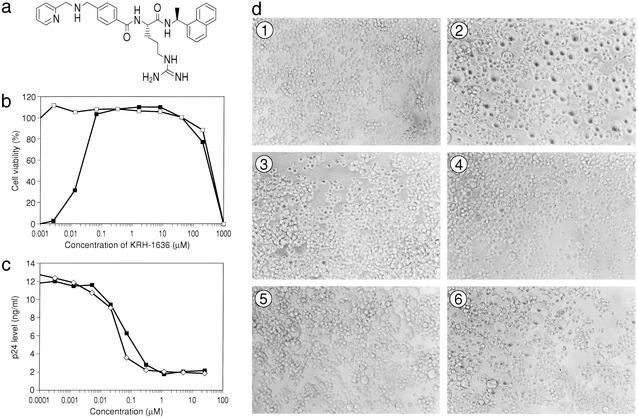
Figure 1.
The chemical structure of the CXCR4 antagonist, KRH-1636, and its anti-HIV-1 activity. (a) The chemical structure of KRH-1636. (b) Anti-HIV-1 activity of KRH-1636 in MT-4 cells as measured by MTT assay. HTLV-IIIB HIV-1 was used in this study (–□–, mock-infected; –■–, HIV-1). (c) Anti-HIV-1 (NL4-3) activity of KRH-1636 in anti-CD3/CD28-stimulated PBMCs as determined by p24 assay. –■–, KRH-1636; –⋄–, AMD3100. (d) Inhibition of cell fusion by KRH-1636. ①, MOLT-4 cells; ②, coculture; ③, 5 μM KRH-1636; ④, 0.5 μM KRH-1636; ⑤, 0.05 μM KRH-1636; ⑥, 0.005 μM KRH-1636.
Cells.
MT-4, MOLT-4, and MOLT-4/HIV-1 human T cell leukemia virus (HTLV)-IIIB cells were maintained in RPMI medium 1640 supplemented with 10% FCS and antibiotics (100 μg/ml penicillin/100 μg/ml streptomycin). Chemokine receptor-expressing human osteosarcoma (HOS) cells were kindly provided by H. Hoshino (Gunma University, Maebashi, Japan) and maintained in DMEM supplemented with 10% FCS and antibiotics. Peripheral blood mononuclear cells (PBMCs) from HIV-1-seronegative healthy donors were isolated by density gradient centrifugation, grown in RPMI medium 1640 supplemented with 10% FCS, and activated with either 1 μg/ml phytohemagglutinin (PHA, Sigma–Aldrich) for 3 days or anti-CD3/CD28 monoclonal antibodies in the presence of IL-2 (20 units/ml, Shionogi, Osaka) at 37°C.
Viruses.
The X4 HIV-1 strains IIIB and NL4-3 were obtained from the culture supernatant of MOLT-4/HTLV-IIIB cells and COS-1 cells transfected with a molecular clone, respectively, as described (18, 19). Aliquots of the viral stocks were stored at −80°C until use. The titer of the virus stocks was determined by endpoint titration of 5-fold limiting dilution in quadruplicate on MT-4 cells. Several clinical isolates including X4 (YU-6, YU-10, and YU-11) (20) and R5 strains (YU-1 and YU-2) (21) were propagated in peripheral blood lymphocytes (PBLs) after PHA stimulation.
Immunofluoresence.
CD4+ T cells from a normal donor were activated with anti-CD3/CD28 monoclonal antibody-bound magnetic beads in human recombinant IL-2 (rIL-2)/10% FCS containing RPMI medium 1640 for 7 days. These activated cells were treated with 10 μM AMD3100 or KRH-1636 in the IL-2-containing medium at 37°C for 1 h. After washing with cold PBS containing 2% FCS and 0.1% NaN3 [fluorescence-activated cell sorting (FACS) buffer], the cells were pretreated with normal human IgG at 0.1 mg/ml in FACS buffer for 15 min on ice to block the Fc receptors and then stained directly with 12G5-phycoerythrin (PharMingen) for 30 min or indirectly with rat IgG1 monoclonal antibodies A145, A80 (22) and T227 at 5 μg/ml for 30 min followed by goat anti-rat IgG-FITC (DAKO) treatment for 30 min on ice. After washing, the cells were fixed with 1% paraformaldehyde in FACS buffer for 5 min at room temperature and then analyzed on FACSCalibur (Becton Dickinson), a flow cytometer, with CELLQUEST software (Becton Dickinson). The area of positivity was determined by using an isotype-matched mouse monoclonal antibody (Beckman Coulter) and a rat monoclonal antibody against hepatitis C virus, Mo8 of IgG1 isotype (23).
In Vitro Anti-HIV-1 Assays.
The 3-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT, Sigma–Aldrich) assay using MT-4 cells was carried out as described (24, 25). For the p24 accumulation assays, PHA-stimulated PBMCs were infected with HIV-1 in the presence of various concentrations of the test compounds unless otherwise stated (16).
Fusion Assay.
MOLT-4 and MOLT-4/HIV-1 HTLV-IIIB cells were cocultured for 1 day as described (26).
DNA Construct and Transfection.
Human cDNAs for CCR3 (27), CCR4 (28), CCR5 (29), and CXCR1 (30) were amplified by PCR from a human PHA-stimulated PBMC cDNA library and cloned into the pcDNA3.1(+) expression vector with cytomegalovirus promoter (Invitrogen). Transfection was performed with Chinese hamster ovary (CHO) cells by using Lipofectamine (Life Technologies, Grand Island, NY), and stable transfectants were selected in the presence of 600 μg/ml geneticin (Life Technologies).
Ligand-Binding Assays.
MT-4, CHO, or chemokine receptor-expressing CHO cells (5 × 106 cells per 0.2 ml per well) were cultured in a 24-well microtiter plate. After 24 h of incubation at 37°C, culture medium was replaced with binding buffer (RPMI medium 1640 supplemented with 0.1% BSA). Binding reactions were performed on ice for 2 h in the presence of [125I]SDF-1α [Daiichi Kagaku Yakuhin, Tokyo; specific activity, 2,200 Ci/mmol (1 Ci = 37 GBq)] and various concentrations of the test compound. The binding reaction was terminated by washing out the free ligand with cold PBS, and the cell-associated radioactivities were counted by using a scintillation counter (Packard Japan, Tokyo).
Coreceptor-Mediated Ca2+ Signaling.
Fura2-acetoxymethyl ester-loaded HOS/CXCR4 cells were incubated in the absence or presence of various concentrations of KRH-1636. Changes in the intracellular Ca2+ level in response to SDF-1α (1 μg/ml) were determined by using a fluorescence spectrophotometer.
Infection of Human PBL/Severe Combined Immunodeficiency (SCID) Mice.
Female C.B-17 SCID mice were purchased from CLEA Japan (Tokyo). i.p. and intrasplenic cell transfers were performed on 6- to 8-week-old mice. SCID mice were depleted of natural killer cells by i.p. injection with TMβ-1 rat anti-mouse IL-2Rβ monoclonal antibody (31) (1 mg per animal). After 1 day, 1 × 107 human PBMCs were introduced into the peritoneum together with human rIL-4 (0.4 μg per animal; PeproTech, Rocky Hill, NJ). On the next day, KRH-1636 (0.1 ml of 2 mM solution per animal) or control (0.1 ml of medium alone) was administered i.p. The NL4-3 strain of HIV-1 (0.2 ml of 1 × 104 tissue culture 50% infective dose per milliliter of stock) was infected i.p. On days 1–7 after infection, the same dosage of KRH-1636 or medium was administered i.p. On days 1, 3, 5, and 7 after infection, 0.4 μg of rIL-4 was also administered i.p. On day 9 after infection, plasma and peritoneal lavage fluid (4 ml) were collected and assayed for HIV-1 p24 with an ELISA kit. At the same time, CD4+ and CD8+ T cell numbers were counted.
For the intrasplenic inoculation, 1.5 × 106 PBMCs were transplanted into spleen together with human rIL-4 (0.4 μg) 2 days after TMβ-1 treatment. On the same day, KRH-1636 (0.1 ml of 2 mM solution per animal) or control (0.1 ml of medium alone) was injected i.p. After 1 day, mice were challenged i.p. with the NL4-3 strain of HIV-1 (0.2 ml of 1 × 104 tissue culture 50% infective dose per milliliter of stock). On days 1–4 after infection, mice were administered i.p. with repeated doses of KRH-1636 or medium. On day 4 after infection, 0.2 μg of rIL-4 was also inoculated i.p. On day 12 after infection, plasma and peritoneal lavage fluid (4 ml) were collected and assayed for HIV-1 p24.
A short-term in vivo test was performed. Mice were treated with TMβ-1. Twenty-four hours later, they were treated with KRH-1636 (0.2 ml i.p.) for 30 min. Animals then were administered i.p. with 4 × 106 PBMCs activated with anti-CD3/anti-CD28 antibody for 6 days and subsequently infected with HIV-1 (1–2 × 103 tissue culture 50% infective dose per 0.1–0.2 ml i.p.) for 2 h. Cells in peritoneal lavage fluid were collected and cultured in IL-2 (20 units/ml)-containing medium. Nine days after culture, p24 assay was performed on culture supernatants.
Detection of KRH-1636 in the Blood After Intraduodenal Administration.
Male Wistar rats (6 weeks old, CLEA Japan) were used for in vivo studies. KRH-1636 or methansulfonated KRH-1636 was dissolved in 45% hydroxypropyl-β-cyclodextrin. After acclimation for at least 1 week, the rats were fasted for ≈18 h before KRH-1636 administration, anesthetized with diethyl ether, and then pylorus ligated. KRH-1636 was administered into the duodenum through a tube at the dose of 200 mg/ml per kg (two rats for KRH-1636 and three rats for methansulfonated KRH-1636). Blood samples were taken from cervic-venous by using a heparin-treated syringe at 0.25, 0.5, 1, 2, 4, and 6 h after administration and centrifuged to separate the plasma. Next, 100 μl of the plasma was mixed with 400 μl of 0.1% formic acid/methanol. After centrifugation (Kubota, Tokyo) at 15,000 rpm for 5 min, the supernatant was evaporated to dryness under the stream of nitrogen, and samples were dissolved in 25% acetonitrile/0.1% formic acid solution and analyzed by the liquid chromatography mass spectroscope QP8000α (Shimadzu). Anti-HIV-1 activity in serial diluted rat serum with complete medium was measured by an MTT assay using MT-4 cells.
Results
KRH-1636 Is a Potent and Selective Inhibitor of X4 HIV-1.
More than 1,000 compounds from the chemical library of Kureha Chemical Industry were surveyed for anti-HIV-1 activity by the conventional MTT assay using CXCR4+MT-4 cells. Through optimization of the lead compound, we found KRH-1636 (Fig. 1a) to be an extremely potent inhibitor of HIV-1 replication in MT-4 cells. The chemistry will be described elsewhere. KRH-1636 completely inhibited X4 HIV-1 (IIIB strain) replication in MT-4 cells at a concentration as low as 0.06 μM (Fig. 1b). Its 50% and 90% effective concentrations (EC50 and EC90) were 0.0193 and 0.0478 μM, respectively. The 50% cytotoxic concentration (CC50) of KRH-1636 was 406.21 μM when MT-4 cells were used. Thus, the selectivity index (ratio of CC50 to EC50) of KRH-1636 was >10,000, indicating that this compound is extremely potent and selective.
The inhibitory activity of KRH-1636 against X4 HIV-1 (NL4-3) replication in PBMCs was also demonstrated by a p24 accumulation assay of culture supernatants of the cells infected with the virus. KRH-1636 (0.0003–20 μM) reduced the antigen levels in a dose-dependent fashion at any time point (Fig. 1c). KRH-1636 was also effective against HTLV-IIIB and three clinical isolates including YU-6 (20), YU-10, and YU-11 and showed a similar potency of action to that observed with the NL4-3 strain (Table 1). In contrast, KRH-1636 was ineffective against R5 HIV-1, YU-1, YU-2 (ref. 21; Table 1), and envelope-modified NL4-3 (data not shown) infection in PBMCs. The specific CXCR4 inhibitors, T22 and AMD3100, completely inhibited replication of the IIIB strain at 0.3 and 0.28 μM, respectively, although they did not inhibit replication of the R5 HIV-1 Ba-L strain at concentrations up to 100 μM (data not shown).
Table 1.
Anti-HIV-1 activity of KRH-1636 in PBMCs
| Virus | Tropism | Compound | EC50, μM |
|---|---|---|---|
| NL4-3 | X4 | KRH-1636 | 0.042 |
| AMD3100 | 0.022 | ||
| HTLV-III B | X4 | KRH-1636 | 0.018 |
| AMD3100 | 0.016 | ||
| YU-6 | X4 | KRH-1636 | 0.046 |
| YU-10 | X4 | KRH-1636 | 0.152 |
| YU-11 | X4 | KRH-1636 | 0.025 |
| YU-1 | R5 | KRH-1636 | >10 |
| YU-2 | R5 | KRH-1636 | >2 |
PHA-stimulated PBMCs were infected with X4- or R5-type HIV-1 strains in the presence of various concentrations of compounds. p24 was monitored 5 days after infection.
KRH-1636 apparently inhibited syncytium formation between MOLT-4 and its HIV-1-converted MOLT-4 cells (Fig. 1d). This indicated that KRH-1636 exerted its effect at the initial step of HIV-1 infection such as viral entry and membrane fusion in the target cells.
All these data show that the effect of KRH-1636 is very similar to that of T22 and AMD3100 and strongly suggest that KRH-1636 might interfere with CXCR4 function as a coreceptor.
KRH-1636 Inhibits Ligand Binding to CXCR4.
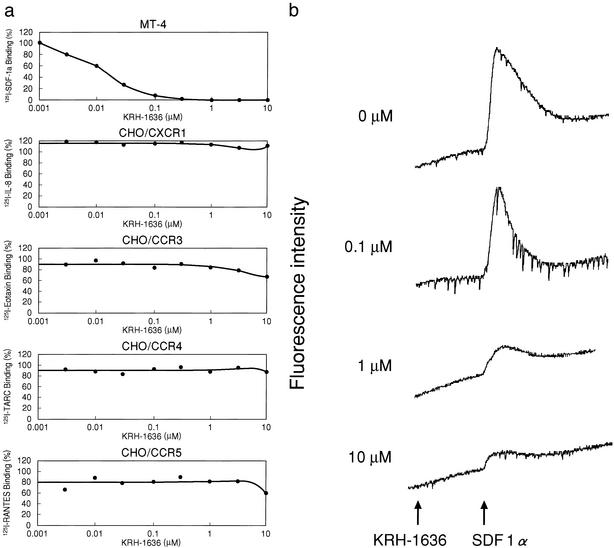
To investigate whether KRH-1636 exerts its effect as a CXCR4 antagonist, the inhibitory effect of the compound on ligand binding to the coreceptor was studied by using MT-4 cells, which express CXCR4 spontaneously. The result showed that [125I]SDF-1α binding was efficiently and dose-dependently suppressed, showing the IC50 of KRH-1636 (Fig. 2a) and control AMD3100 to be 0.013 μM and 0.068 μM, respectively. To determine whether the inhibitory effect of KRH-1636 on chemokine binding is specific to CXCR4, the activity of KRH-1636 was examined in CHO cells stably expressing CXCR1, CCR3, CCR4, or CCR5. This compound had no effect on the binding of [125I]IL-8, [125I]eotaxin, [125I]TARC (thymus- and activation-regulated chemokine), or [125I]RANTES to CXCR1, CCR3, CCR4, and CCR5, respectively (Fig. 2a). These data clearly demonstrate that KRH-1636 selectively inhibits the binding of SDF-1α to CXCR4.
Figure 2.
Inhibitory effects of KRH-1636 on chemokine binding to the cells and SDF-1α-induced Ca2+ mobilization in HOS/CXCR4 cells. (a) Inhibitory effects of KRH-1636 on chemokine binding to MT-4 or CXCR1-, CCR3-, CCR4-, or CCR5-expressing CHO cells. The percent binding was calculated as 100 × [(binding with inhibitor − nonspecific binding)/(binding without inhibitor − nonspecific binding)]. TARC, thymus- and activation-regulated chemokine. (b) Inhibitory effect of KRH-1636 on SDF-1α-induced Ca2+ mobilization in HOS/CXCR4 cells.
KRH-1636 Inhibits CXCR4-Mediated Ca2+ Signaling.
In chemokine-induced Ca2+-mobilization experiments, SDF-1α clearly increased the intracellular Ca2+ level in HOS/CXCR4 cells at the concentration of 1 μg/ml (Fig. 2b). The addition of 10 μM KRH-1636 did not affect the Ca2+ level but strongly abrogated the SDF-1α-induced increase in the intracellular Ca2+ level of HOS/CXCR4 cells, and the effect was dose-dependent (Fig. 2b). In contrast, this compound did not affect the Ca2+ mobilization induced by RANTES, MCP-1, eotaxin, macrophage-derived chemokine, and MIP-1α in HOS/CCR1, HOS/CCR2b, HOS/CCR3, HOS/CCR4, and HOS/CCR5 cells, respectively, even at the concentration of 10 μM (data not shown). These results indicate that KRH-1636 selectively blocks CXCR4-mediated Ca2+ signaling.
Interaction of KRH-1636 with CXCR4.
We used four different kinds of monoclonal antibodies, 12G5 [anti-CXCR4, HIV-neutralizing, recognizing its extracellular loop (ECL)1 and -2], A145 (anti-CXCR4, recognizing its N terminus), A80 (anti-CXCR4, HIV-enhancing, recognizing its ECL3), and T227 (anti-CCR5), to study the interaction of KRH-1636 with CXCR4. The binding of 12G5 and A80 but not A145 and T227 monoclonal antibodies to CD3-activated T cells was inhibited dramatically by KRH-1636 (Fig. 4, which is published as supporting information on the PNAS web site, www.pnas.org). Interestingly, the control antagonist AMD3100 showed a similar result. From these data it is strongly suggested that KRH-1636 inhibited the binding of 12G5 and A80 to CXCR4 without inducing down-modulation of the CXCR4 molecule or affecting CCR5 expression.
KRH-1636 Blocks X4 HIV-1 Replication in Human PBL/SCID Mice.
KRH-1636 was examined to determine whether it inhibits X4 viral replication in vivo. For this, we used both long-term and short-term in vivo assays with human PBL/SCID mice. For the long-term tests we applied two different systems in which human PBL/SCID mice were constructed by either an i.p. or intrasplenic inoculation of PBMCs. Because the effect of X4 virus (NL4-3) infection is not as clear as that of R5 virus infection in human PBL/SCID mice, C.B-17 mice were treated with human rIL-4 during the infection period to enhance the replication of X4 HIV-1 (see Materials and Methods). The amounts of p24 in peritoneal lavage fluid in five of seven animals with PBMCs that were inoculated i.p. were below the detectable level in KRH-1636-treated mice 9 days after infection, whereas viremia was noted in all seven untreated mice (Table 2). However, a decrease in HIV-1-infected CD4+ T cell number was not clear in untreated mice under the present experimental condition (data not shown). Similarly, the amounts of p24 in the plasma and peritoneal lavage in two animals with PBMCs that were given intrasplenically were below the detectable level in KRH-1636-treated mice after infection, whereas viremia was noted in three untreated mice (data not shown). In a short-term in vivo test, KRH-1636 clearly inhibited viral replication and HIV-1-induced CD4+ T cell decrease as observed in the in vitro assays (Table 3).
Table 2.
Anti-HIV-1 effects of KRH-1636 in human PBL-SCID mice as assessed by the long-term in vivo test
| Mouse | Infected with | Treated with | P24 level, pg/ml
|
|
|---|---|---|---|---|
| Plasma | P.L.* | |||
| 1 | Mock | Medium | <2 | <2 |
| 2 | Mock | KRH-1636† | <2 | <2 |
| 3 | NL4-3 | Medium | <2 | 4.1 |
| 4 | NL4-3 | Medium | <2 | 5.6 |
| 5 | NL4-3 | Medium | 8.4 | 28.2 |
| 6 | NL4-3 | Medium | 12.9 | 14.9 |
| 7 | NL4-3 | Medium | 10.3 | 180.2 |
| 8 | NL4-3 | Medium | 4.4 | 20.7 |
| 9 | NL4-3 | Medium | <2 | 8.5 |
| 10 | NL4-3 | KRH-1636 | <2 | <2 |
| 11 | NL4-3 | KRH-1636 | <2 | <2 |
| 12 | NL4-3 | KRH-1636 | <2 | <2 |
| 13 | NL4-3 | KRH-1636 | <2 | <2 |
| 14 | NL4-3 | KRH-1636 | <2 | <2 |
| 15 | NL4-3 | KRH-1636 | 6.9 | 14.6 |
| 16 | NL4-3 | KRH-1636 | 17.8 | 29.5 |
Mice were inoculated with PBMCs intraperitoneally.
Peritoneal lavage.
2 mM, 0.1 ml.
Table 3.
Anti-HIV-1 effects of KRH-1636 in human PBL-SCID mice as assessed by the short-term in vivo test
| Mouse | Treated with | CD4+T cells (×104) | p24 level, pg/ml |
|---|---|---|---|
| 1 | Medium | 1.3 | 107 |
| 2 | Medium | 4.4 | 2,295 |
| 3 | Medium | 3.8 | 1,326 |
| 4 | KRH-1636* | 37.5 | <5 |
| 5 | KRH-1636 | 0.8 | <5 |
| 6 | KRH-1636 | 10.1 | <5 |
| 7 | KRH-1636 | 17.1 | <5 |
| 8 | KRH-1636 | 60.2 | <5 |
Cells in peritoneal lavage fluid were collected and cultured in IL-2-containing medium, and a p24 assay was performed 9 days after culture.
0.1 mM, 0.2 ml.
KRH-1636 Is Absorbed Efficiently from the Duodenum into the Blood.
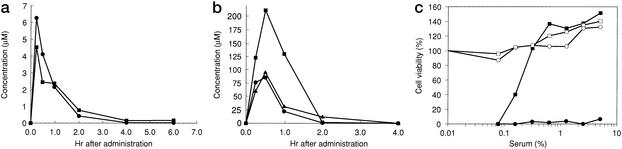
Finally, the absorbability of KRH-1636 was investigated by using rats as described in Materials and Methods. The concentration of KRH-1636 in plasma or serum was monitored by liquid chromatography MS (plasma) and anti-HIV-1 activity (serum) after intraduodenal administration. To increase the drug availability, the methansulfonated analog of KRH-1636 was also synthesized and studied in a similar manner. The results showed that these compounds were absorbed efficiently from the duodenum of the rats (Fig. 3, a for KRH-1636 and b for methansulfonated KRH-1636). The bioavailability of KRH-1636 and methansulfonated KRH-1636 in this experiment was estimated to be ≈7% and 69%, respectively, by liquid chromatography MS. Moreover, EC50 (%) and EC90 (%) of serum at 60 min after the administration of KRH-1636 was 0.18 and 0.27, respectively, as determined by the MTT assay using MT-4 cells (Fig. 3c). In this experiment, the concentration of KRH-1636 in plasma at 60 min after administration was 30.6 μM. Therefore, the concentration of KRH-1636 in the blood reached levels that are inhibitory to HIV replication.
Figure 3.
Absorbability of KRH-1636 and methansulfonated KRH-1636 after intraduodenal administration into Wistar rats. Two and three rats were administered with KRH-1636 (a) and methansulfonated KRH-1636 (b), respectively. Each symbol represents the data from each animal. (c) The MTT assay was carried out by using MT-4 cells to determine the EC50 (%) and EC90 (%) of serum at 60 min after the administration of KRH-1636. –□–, KRH-1636/mock; –■–, KRH-1636/HIV-1; –○–, control/mock; –●–, control/HIV-1.
Discussion
This report demonstrates a duodenally absorbable CXCR4 antagonist, KRH-1636, that has a potent anti-HIV activity both in vivo and in vitro.
KRH-1636 selectively inhibited infection of T cell line-tropic X4 virus strains including several clinical isolates without affecting R5 HIV-1. Macrophage-tropic R5 virus strains are isolated from individuals shortly after seroconversion and, during the asymptomatic period of the disease, seem to be responsible for sexual and parenteral transmission and represent the most prevalent phenotype (32, 33). In contrast, X4 HIV-1 isolates tend to appear in infected individuals at the later stages of infection during transition from the asymptomatic to symptomatic state and may be involved in the rapid decline of CD4+ T lymphocytes and progression to AIDS due to strong cytopathicity (34). It therefore is important to find therapies to inhibit CXCR4-mediated infection by X4 HIV-1 to block progression to AIDS.
The inhibition of chemokine receptors by KRH-1636 seemed to be specific to CXCR4 because KRH-1636 was highly inhibitory to the binding of [125I]SDF-1α to MT-4 cells (Fig. 2a). Furthermore, chemokines binding to CXCR1, CCR3, CCR4, or CCR5 was not inhibited by this compound. Therefore, the inhibitory effect of KRH-1636 was shown to be selective for CXCR4. The inhibitory effects of KRH-1636 on the binding of monoclonal antibodies to CXCR4 (Fig. 4) also support this notion. The specificity of KRH-1636 to CXCR4 seems extremely important from a chemotherapeutic viewpoint, because nonspecific inhibition of β-chemokine receptors may generate serious side effects associated with chemokine dysregulation.
Similar to AMD3100, KRH-1636 did not induce the internalization of CXCR4 (Fig. 4). However, KRH-1636 suppressed the binding of the anti-CXCR4 antibodies, A80 and 12G5, to activated CD4+ T cells. Because these monoclonal antibodies are directed to the different ECLs of the seven-transmembrane receptor CXCR4, it is possible that KRH-1636 may interact with all three ECLs. Alternatively, KRH-1636 may induce a conformational change in the CXCR4 after binding to a distinct site of coreceptor. The exact site on the CXCR4 molecule targeted by this compound remains to be determined.
Oral availability remains one of the key goals to be attained as an anti-HIV drug, because retroviral infection continues throughout the life of infected people. Thus, oral administration of KRH-1636 was conducted in rats and SCID mice (data not shown), the plasma concentrations of the compound and pharmacokinetic parameters of which were determined. Although no measurable absorption of the currently available chemokine receptor antagonists has been reported, substantial transfer not only as a chemical but also as an active anti-HIV compound from the gastrointestinal tract to the blood was achieved with KRH-1636. Further improvement of oral availability and increased penetration into the central nervous system is needed.
C.B-17 SCID mice exhibit multiple immunological defects, and it has been demonstrated that this mutant strain efficiently receives human PBLs (35). After infection, R5-type HIV-1, including JR-CSF and NFN-SX strains, could replicate very efficiently in association with the apparent CD4+ T cell depletion. However, X4 virus does not replicate well in this system, and therefore CD4+ T cell decline is not obvious (36). To overcome this problem, the mice were treated with human IL-4 because our preliminary result showed that X4 virus strains favor the T helper 2-dominant status for their replication and induction of CD4+ T cell decline (37). Our studies clearly showed the in vivo inhibitory effect of KRH-1636 on the replication of X4 virus. Although this study was conducted by using an i.p. injection of the compound, oral administration should be effective, because oral bioavailability and efficient adsorption of this compound have been confirmed in rats and the SCID mouse system as well.
Several questions remain to be answered concerning the clinical outcome of coreceptor-based therapy (38). Will blocking of normal CXCR4 function caused by treatment with antagonists be tolerated? In the case of CCR5 antagonists, it will be safe because of the lack of reported immunological disorders in individuals with CCR5Δ32 homozygotes. However, inhibition of SDF-1α–CXCR4 interactions may be more problematic, because knockout of either the SDF-1α or CXCR4 gene in mice causes severe defects including abnormal hematopoiesis, cerebellar development, and vascularization of the gastrointestinal tract (39–41). Should not the emergence of drug resistance be regarded as a serious matter? It is unlikely that frequent mutations occur in the second ECL of CXCR4. However, HIV-1 may be able to acquire resistance to KRH-1636 by amino acid mutations of the viral envelope protein gp120. In fact, an X4 HIV-1 resistant to SDF-1α and AMD3100 has been reported recently (42). The resistant virus had multiple mutations in gp120 but did not switch chemokine receptor usage. These and related questions will hopefully be answered as coreceptor-based therapies proceed to clinical trials. Acute toxicological studies have indicated the absence of severe toxicity in rats that received KRH-1636 (1.5 mg/kg per day) by i.v. administration for 4 days (data not shown).
In conclusion, KRH-1636, which can be absorbed from the duodenum into the blood, seems to be a promising agent for the treatment and prophylaxis of HIV-1 infection.
Supplementary Material
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, the Ministry of Health, Labor, and Welfare of Japan, and Core Research for Evolutional Science and Technology of Japan.
Abbreviations
- HIV-1
HIV type 1
- CCR
CC chemokine receptor
- CXCR
CXC chemokine receptor
- SDF-1α
stromal cell-derived factor 1α
- HOS
human osteosarcoma
- HTLV
human T cell leukemia virus
- PBMC
peripheral blood mononuclear cell
- PHA
phytohemagglutinin
- PBL
peripheral blood lymphocyte
- rIL-2
recombinant IL-2
- MTT
3-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan
- CHO
Chinese hamster ovary
- SCID
severe combined immunodeficiency
- ECL
extracellular loop
References
- 1.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, et al. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 2.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Jr, Feinberg J E, Balfour H H, Jr, Deyton L R, et al. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 3.Palella F J, Jr, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Cameron D W, Heath-Chiozzi M, Danner S, Cohen C, Kravcik S, Maurath C, Sun E, Henry D, Rode R, Potthoff A, Leonard J. Lancet. 1998;351:543–549. doi: 10.1016/s0140-6736(97)04161-5. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 6.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, et al. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 7.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 8.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 9.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 10.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, et al. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 11.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 12.Schols D, Struyf S, Van Damme J, Este J A, Henson G, De Clercq E. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donzella G A, Schols D, Lin S W, Este J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clercq E, Moore J P. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 14.Hendrix C W, Flexner C, MacFarland R T, Giandomenico C, Fuchs E J, Redpath E, Bridger G, Henson G W. Antimicrob Agents Chemother. 2000;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuda M, Nakashima H, Ueda T, Naba H, Ikoma R, Otaka A, Terakawa Y, Tamamura H, Ibuka T, Murakami T, et al. Biochem Biophys Res Commun. 1992;189:845–850. doi: 10.1016/0006-291x(92)92280-b. [DOI] [PubMed] [Google Scholar]
- 16.Murakami T, Nakajima T, Koyanagi Y, Tachibana K, Fujii N, Tamamura H, Yoshida N, Waki M, Matsumoto A, Yoshie O, et al. J Exp Med. 1997;186:1389–1393. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, Shiraishi M, Aramaki Y, Okonogi K, Ogawa Y, et al. Proc Natl Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada S, Koyanagi Y, Yamamoto N. Virology. 1985;146:272–281. doi: 10.1016/0042-6822(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 19.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshiyama H, Kobayashi N, Matsui T, Nakashima H, Kajii T, Yamato K, Kotani S, Miyoshi I, Yamamoto N. Mol Biol Med. 1987;4:385–396. [PubMed] [Google Scholar]
- 21.Yoshiyama H, Koyanagi Y, Nakashima H, Ishihara T, Uchino F, Harada S, Okino F, Kajii T, Yamamoto N. Jpn J Cancer Res. 1986;77:16–20. [PubMed] [Google Scholar]
- 22.Tanaka R, Yoshida A, Murakami T, Baba E, Lichtenfeld J, Omori T, Kimura T, Tsurutani N, Fujii N, Wang Z X, et al. J Virol. 2001;75:11534–11543. doi: 10.1128/JVI.75.23.11534-11543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inudoh M, Kato N, Tanaka Y. Microbiol Immunol. 1998;42:875–877. doi: 10.1111/j.1348-0421.1998.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E. J Virol Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu N, Naoe T, Kawazoe Y, Sakagami H, Nakashima H, Murakami T, Yamamoto N. Biol Pharm Bull. 1993;16:434–436. doi: 10.1248/bpb.16.434. [DOI] [PubMed] [Google Scholar]
- 26.Chowdhury I H, Koyanagi Y, Kobayashi S, Hamamoto Y, Yoshiyama H, Yoshida T, Yamamoto N. Virology. 1990;176:126–132. doi: 10.1016/0042-6822(90)90237-l. [DOI] [PubMed] [Google Scholar]
- 27.Combadiere C, Ahuja S K, Murphy P M. J Biol Chem. 1995;270:16491–16494. doi: 10.1074/jbc.270.28.16491. [DOI] [PubMed] [Google Scholar]
- 28.Power C A, Meyer A, Nemeth K, Bacon K B, Hoogewerf A J, Proudfoot A E, Wells T N. J Biol Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- 29.Raport C J, Gosling J, Schweickart V L, Gray P W, Charo I F. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 30.Holmes W E, Lee J, Kuang W J, Rice G C, Wood W I. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- 31.Tanaka T, Kitamura F, Nagasaka Y, Kuida K, Suwa H, Miyasaka M. J Exp Med. 1993;178:1103–1107. doi: 10.1084/jem.178.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 33.van't Wout A B, Kootstra N A, Mulder-Kampinga G A, Albrecht-van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. J Clin Invest. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richman D D, Bozzette S A. J Infect Dis. 1994;169:968–974. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 35.Mosier D E, Gulizia R J, Baird S M, Wilson D B, Spector D H, Spector S A. Science. 1991;251:791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 36.Mosier D E, Gulizia R J, MacIsaac P D, Torbett B E, Levy J A. Science. 1993;260:689–692. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y, Koyanagi Y, Tanaka Y, Murakami T, Misawa N, Maeda N, Kimura T, Shida H, Hoxie J A, O'Brien W A, Yamamoto N. J Virol. 1999;73:316–324. doi: 10.1128/jvi.73.1.316-324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schols D, Verh K. Verh K Vlaam Acad Geneeskd Belg. 1999;61:551–564. [PubMed] [Google Scholar]
- 39.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 40.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, et al. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 41.Zou Y R, Kottmann A H, Kuroda M, Taniuchi I, Littman D R. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 42.Schols D, Este J A, Cabrera C, De Clercq E. J Virol. 1998;72:4032–4037. doi: 10.1128/jvi.72.5.4032-4037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.