Abstract
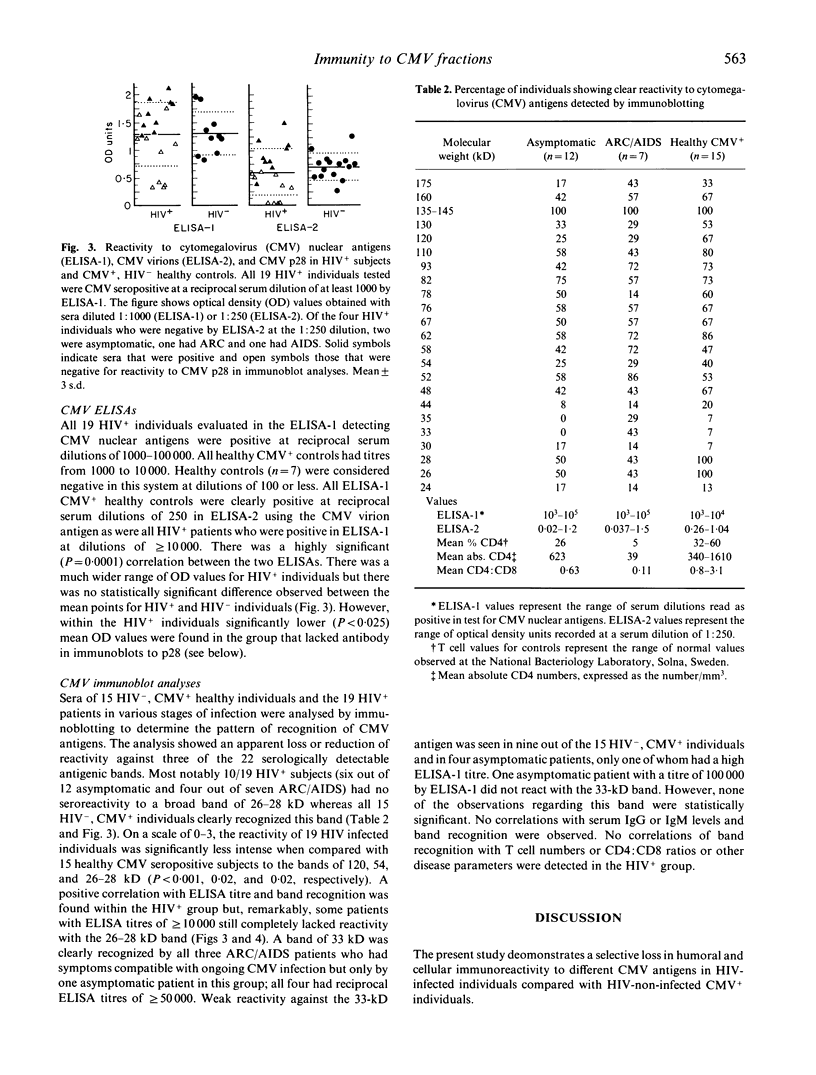
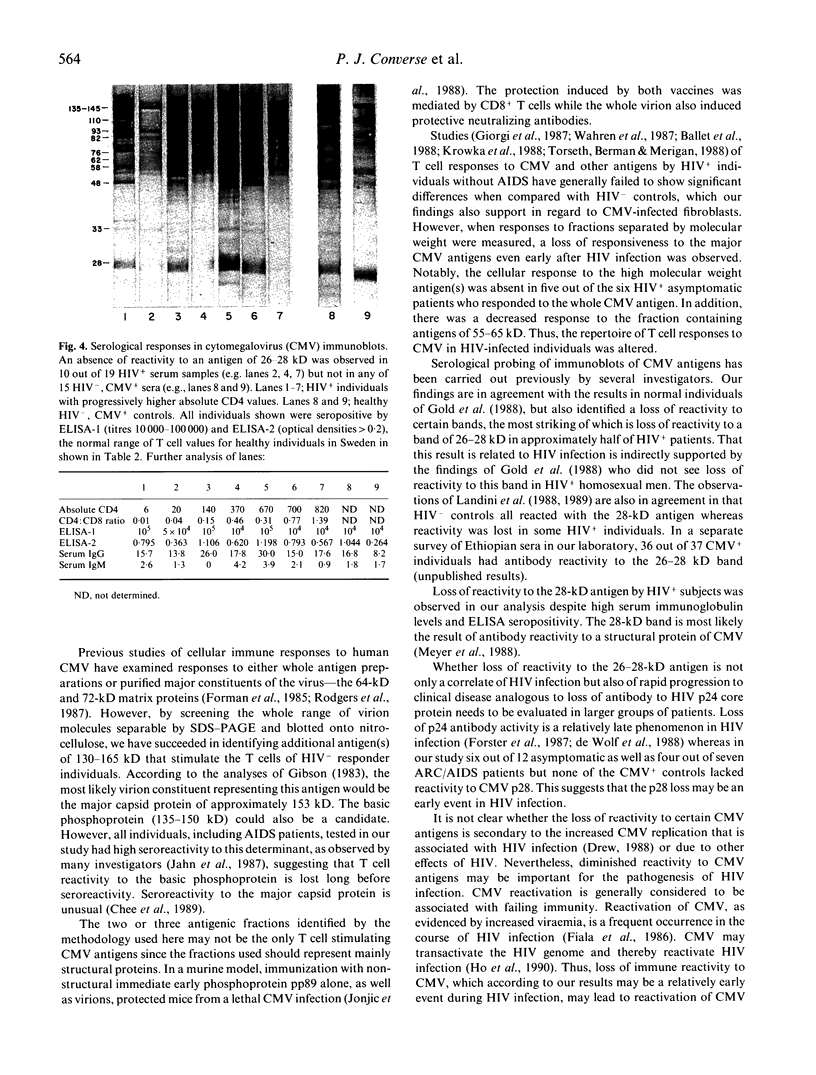
In order to delineate the molecular pathogenesis of the increased susceptibility to CMV disease in HIV infection, the patterns of antigen responsiveness in HIV-infected and non-infected individuals were investigated. CMV was fractionated by SDS-PAGE and electroblotted onto nitrocellulose. Lymphoproliferative responses of healthy HIV-, CMV+ individuals and HIV+, CMV+ asymptomatic patients to a whole CMV antigen preparation and to 20 fractions of nitrocellulose-bound CMV were then compared. Three fractions of approximate molecular weight of 130-165, 65-75, and 55-65 kD appeared to contain the major T cell stimulating antigens for HIV-, CMV+ individuals. A statistically significant depression of responses to fractions containing antigens in the ranges of 130-165 kD and 55-65 kD but not to whole CMV was seen in HIV+ individuals compared with controls. In healthy controls, the sum of the proliferative responses as measured by 3H-thymidine uptake to these three major fractions was approximately equal to the response to a whole CMV antigen preparation, whereas it was less than half of this response in five out of six HIV+ subjects. When antibody activities to CMV antigens were analysed by immunoblotting of sera from the two subject groups and also sera of ARC and AIDS patients, a selective loss of reactivity was revealed in 10 out of 19 HIV+ subjects to a band of 26-28 kD whereas all 15 HIV-, CMV+ controls recognized this band. Serum IgG and IgM values were both significantly higher in HIV+ individuals than in controls. These findings suggest that specific lesions in the repertoire of immune responsiveness to CMV antigens occur in HIV+ individuals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Ballet J. J., Couderc L. J., Rabian-Herzog C., Duval-Roy C., Janier M., Danon F., Clauvel J. P., Seligmann M. Impaired T-lymphocyte-dependent immune responses to microbial antigens in patients with HIV-1-associated persistent generalized lymphadenopathy. AIDS. 1988 Aug;2(4):291–297. doi: 10.1097/00002030-198808000-00009. [DOI] [PubMed] [Google Scholar]
- Chee M., Rudolph S. A., Plachter B., Barrell B., Jahn G. Identification of the major capsid protein gene of human cytomegalovirus. J Virol. 1989 Mar;63(3):1345–1353. doi: 10.1128/jvi.63.3.1345-1353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse P. J., Hess A. D., Tutschka P. J., Santos G. W. Effect of cyclosporine on the response of normal human lymphocytes to cytomegalovirus in vitro. Infect Immun. 1983 Sep;41(3):1226–1233. doi: 10.1128/iai.41.3.1226-1233.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse P. J., Ottenhoff T. H., Gebre N., Ehrenberg J. P., Kiessling R. Cellular, humoral, and gamma interferon responses to Mycobacterium leprae and BCG antigens in healthy individuals exposed to leprosy. Scand J Immunol. 1988 May;27(5):515–525. doi: 10.1111/j.1365-3083.1988.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Drew W. L. Cytomegalovirus infection in patients with AIDS. J Infect Dis. 1988 Aug;158(2):449–456. doi: 10.1093/infdis/158.2.449. [DOI] [PubMed] [Google Scholar]
- Drew W. L., Mintz L., Miner R. C., Sands M., Ketterer B. Prevalence of cytomegalovirus infection in homosexual men. J Infect Dis. 1981 Feb;143(2):188–192. doi: 10.1093/infdis/143.2.188. [DOI] [PubMed] [Google Scholar]
- Fehniger T. E., Mengistu G., Gessesse A., Gabre-Mariam H., Akuffo H. Changes in the antigenic profile of Leishmania parasites following shifts in temperature. Acta Trop. 1990 May;47(4):227–236. doi: 10.1016/0001-706x(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Fiala M., Cone L. A., Chang C. M., Mocarski E. S. Cytomegalovirus viremia increases with progressive immune deficiency in patients infected with HTLV-III. AIDS Res. 1986 Summer;2(3):175–181. doi: 10.1089/aid.1.1986.2.175. [DOI] [PubMed] [Google Scholar]
- Forman S. J., Zaia J. A., Clark B. R., Wright C. L., Mills B. J., Pottathil R., Racklin B. C., Gallagher M. T., Welte K., Blume K. G. A 64,000 dalton matrix protein of human cytomegalovirus induces in vitro immune responses similar to those of whole viral antigen. J Immunol. 1985 May;134(5):3391–3395. [PubMed] [Google Scholar]
- Forster S. M., Osborne L. M., Cheingsong-Popov R., Kenny C., Burnell R., Jeffries D. J., Pinching A. J., Harris J. R., Weber J. N. Decline of anti-p24 antibody precedes antigenaemia as correlate of prognosis in HIV-1 infection. AIDS. 1987 Dec;1(4):235–240. [PubMed] [Google Scholar]
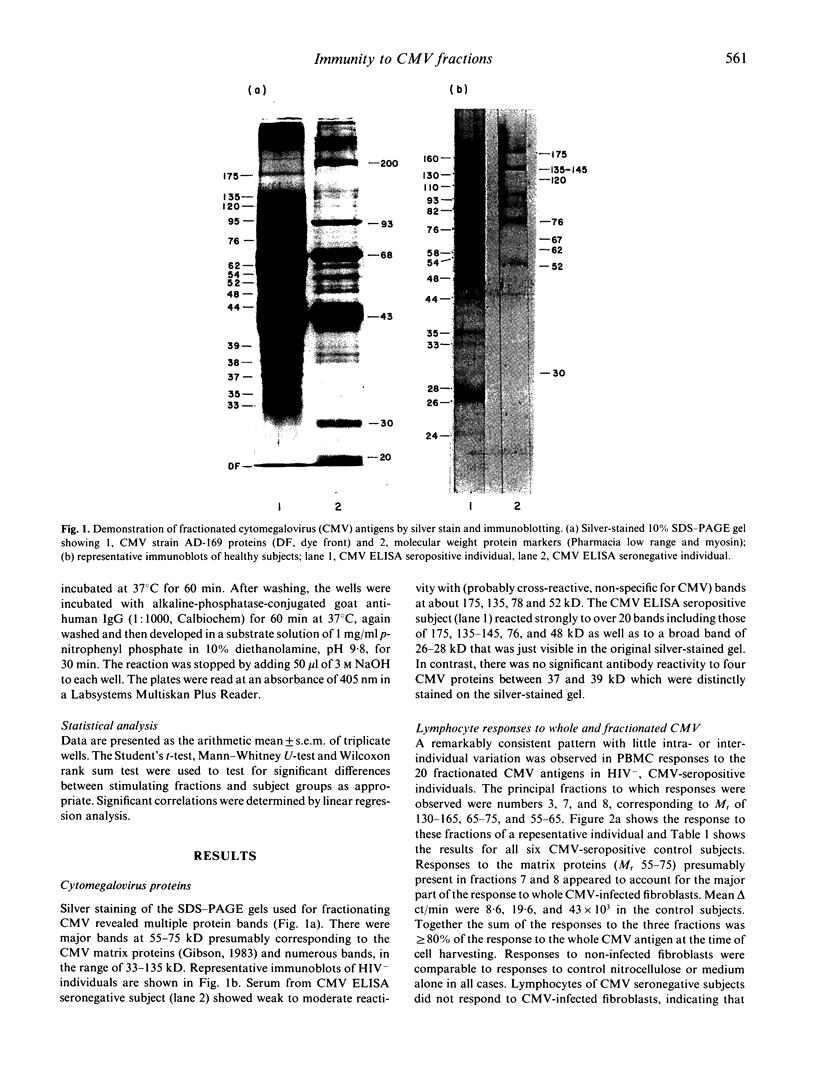
- Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983 Jul 30;128(2):391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- Giorgi J. V., Fahey J. L., Smith D. C., Hultin L. E., Cheng H. L., Mitsuyasu R. T., Detels R. Early effects of HIV on CD4 lymphocytes in vivo. J Immunol. 1987 Jun 1;138(11):3725–3730. [PubMed] [Google Scholar]
- Gold D., Ashley R., Handsfield H. H., Verdon M., Leach L., Mills J., Drew L., Corey L. Immunoblot analysis of the humoral immune response in primary cytomegalovirus infection. J Infect Dis. 1988 Feb;157(2):319–326. doi: 10.1093/infdis/157.2.319. [DOI] [PubMed] [Google Scholar]
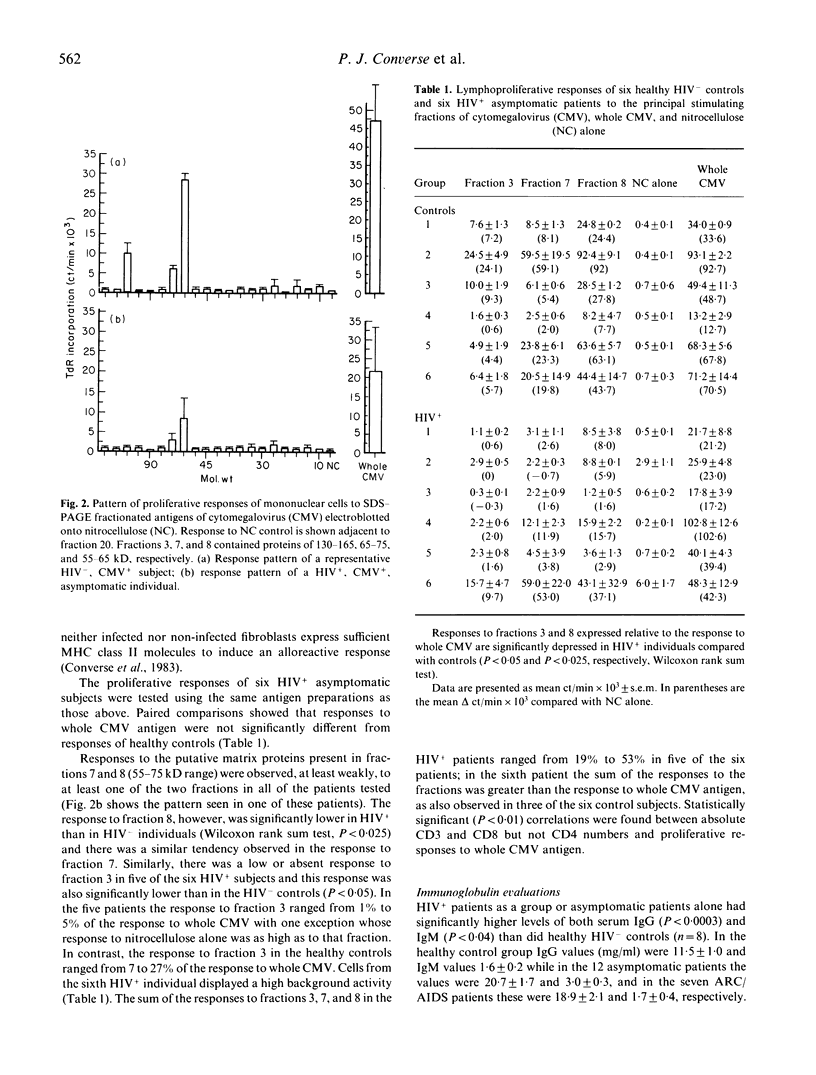
- Ho W. Z., Harouse J. M., Rando R. F., Gönczöl E., Srinivasan A., Plotkin S. A. Reciprocal enhancement of gene expression and viral replication between human cytomegalovirus and human immunodeficiency virus type 1. J Gen Virol. 1990 Jan;71(Pt 1):97–103. doi: 10.1099/0022-1317-71-1-97. [DOI] [PubMed] [Google Scholar]
- Jahn G., Scholl B. C., Traupe B., Fleckenstein B. The two major structural phosphoproteins (pp65 and pp150) of human cytomegalovirus and their antigenic properties. J Gen Virol. 1987 May;68(Pt 5):1327–1337. doi: 10.1099/0022-1317-68-5-1327. [DOI] [PubMed] [Google Scholar]
- Jonjić S., del Val M., Keil G. M., Reddehase M. J., Koszinowski U. H. A nonstructural viral protein expressed by a recombinant vaccinia virus protects against lethal cytomegalovirus infection. J Virol. 1988 May;62(5):1653–1658. doi: 10.1128/jvi.62.5.1653-1658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krowka J., Stites D., Mills J., Hollander H., McHugh T., Busch M., Wilhelm L., Blackwood L. Effects of interleukin 2 and large envelope glycoprotein (gp 120) of human immunodeficiency virus (HIV) on lymphocyte proliferative responses to cytomegalovirus. Clin Exp Immunol. 1988 May;72(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Landini M. P., Baldassarri B., Mirolo G., Ripalti A., La Placa M. Reactivity of cytomegalovirus structural polypeptides with different subclasses of IgG present in human serum. J Infect. 1988 Mar;16(2):163–167. doi: 10.1016/s0163-4453(88)93997-7. [DOI] [PubMed] [Google Scholar]
- Landini M. P., Mirolo G., Re M. C., Ripalti A., La Placa M. Antibody reactivity to cytomegalovirus structural polypeptides in subclinical and clinical human immunodeficiency virus infections. Eur J Clin Microbiol Infect Dis. 1989 Feb;8(2):159–163. doi: 10.1007/BF01963904. [DOI] [PubMed] [Google Scholar]
- Landini M. P., Re M. C., Mirolo G., Baldassarri B., La Placa M. Human immune response to cytomegalovirus structural polypeptides studied by immunoblotting. J Med Virol. 1985 Dec;17(4):303–311. doi: 10.1002/jmv.1890170403. [DOI] [PubMed] [Google Scholar]
- Meyer H., Bankier A. T., Landini M. P., Brown C. M., Barrell B. G., Rüger B., Mach M. Identification and procaryotic expression of the gene coding for the highly immunogenic 28-kilodalton structural phosphoprotein (pp28) of human cytomegalovirus. J Virol. 1988 Jul;62(7):2243–2250. doi: 10.1128/jvi.62.7.2243-2250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. D., Leszczynski J., Zaia J. A., Flournoy N., Newton B., Snydman D. R., Wright G. G., Levin M. J., Thomas E. D. Prevention of cytomegalovirus infection by cytomegalovirus immune globulin after marrow transplantation. Ann Intern Med. 1983 Apr;98(4):442–446. doi: 10.7326/0003-4819-98-4-442. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Hillman J. K., Rubin B. Y., Kelly C. D., Jacobs J. L., Tyler L. W., Donelly D. M., Carriero S. M., Godbold J. H., Roberts R. B. Patients at risk for AIDS-related opportunistic infections. Clinical manifestations and impaired gamma interferon production. N Engl J Med. 1985 Dec 12;313(24):1504–1510. doi: 10.1056/NEJM198512123132403. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Rodgers B., Borysiewicz L., Mundin J., Graham S., Sissons P. Immunoaffinity purification of a 72K early antigen of human cytomegalovirus: analysis of humoral and cell-mediated immunity to the purified polypeptide. J Gen Virol. 1987 Sep;68(Pt 9):2371–2378. doi: 10.1099/0022-1317-68-9-2371. [DOI] [PubMed] [Google Scholar]
- Stagno S., Reynolds D. W., Tsiantos A., Fuccillo D. A., Long W., Alford C. A. Comparative serial virologic and serologic studies of symptomatic and subclinical congenitally and natally acquired cytomegalovirus infections. J Infect Dis. 1975 Nov;132(5):568–577. doi: 10.1093/infdis/132.5.568. [DOI] [PubMed] [Google Scholar]
- Sundqvist V. A., Wahren B. An interchangeable ELISA for cytomegalovirus antigen and antibody. J Virol Methods. 1981 Apr;2(5):301–312. doi: 10.1016/0166-0934(81)90029-x. [DOI] [PubMed] [Google Scholar]
- Torseth J. W., Berman P. W., Merigan T. C. Recombinant HIV structural proteins detect specific cellular immunity in vitro in infected individuals. AIDS Res Hum Retroviruses. 1988 Feb;4(1):23–30. doi: 10.1089/aid.1988.4.23. [DOI] [PubMed] [Google Scholar]
- Wahren B., Morfeldt-Månsson L., Biberfeld G., Moberg L., Sönnerborg A., Ljungman P., Werner A., Kurth R., Gallo R., Bolognesi D. Characteristics of the specific cell-mediated immune response in human immunodeficiency virus infection. J Virol. 1987 Jun;61(6):2017–2023. doi: 10.1128/jvi.61.6.2017-2023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A., Lee C. A., Cook D. G., Grundy J. E., Emery V. C., Kernoff P. B., Griffiths P. D. Cytomegalovirus infection and progression towards AIDS in haemophiliacs with human immunodeficiency virus infection. Lancet. 1989 Jul 8;2(8654):63–66. doi: 10.1016/s0140-6736(89)90312-7. [DOI] [PubMed] [Google Scholar]
- Young D. B., Lamb J. R. T lymphocytes respond to solid-phase antigen: a novel approach to the molecular analysis of cellular immunity. Immunology. 1986 Oct;59(2):167–171. [PMC free article] [PubMed] [Google Scholar]
- de Wolf F., Lange J. M., Houweling J. T., Coutinho R. A., Schellekens P. T., van der Noordaa J., Goudsmit J. Numbers of CD4+ cells and the levels of core antigens of and antibodies to the human immunodeficiency virus as predictors of AIDS among seropositive homosexual men. J Infect Dis. 1988 Sep;158(3):615–622. doi: 10.1093/infdis/158.3.615. [DOI] [PubMed] [Google Scholar]