Individuals with Stargardt's disease can navigate a crowd by using peripheral vision, but reading and recognizing faces can be onerous and shapes and contours may appear distorted. This progressive and devastating visual impairment reflects the loss of light-absorbing photoreceptor cells in the central area of the retina (macula), which is typical of this juvenile form of macular degeneration (1). Yet to understand the cause of photoreceptor cell degeneration in Stargardt's macular degeneration it is necessary to take into account the retinal pigment epithelium (RPE), a cell layer lying adjacent to the photoreceptor cells. Because the RPE is vital to the integrity of the photoreceptor cells, the demise of RPE cells brings about the loss of photoreceptors (2).
Studies performed over the past several years have pointed to the fluorophores that constitute the lipofuscin of RPE cells as being crucial factors in the degeneration of these cells in macular degeneration. Of added importance is the fact that lipofuscin is not just a feature of the Stargardt's disease; it also accumulates with age in the RPE cells of all eyes (3). Much of this indigestible pigment originates in the photoreceptor cell, with deposition in the RPE occurring because it is the responsibility of the RPE to internalize membranous debris discarded daily by the photoreceptor cell. Characterization of the composition of RPE lipofuscin has revealed that a major constitutent is A2E, a conjugate of vitamin A aldehyde (4–6). A2E has a pyridinium bisretinoid structure that is unprecedented (7) and once formed it cannot be enzymatically degraded. When amassed to sufficient concentrations, A2E can mediate detergent-like effects on cellular membranes (8, 9), alter lysosomal function (10, 11), and release proapoptotic proteins from mitochondria (12). A2E also confers a susceptibility to blue light-induced RPE cell death, with photooxidative products of A2E being the intermediates that ravage cellular macromolecules (12–15). Given the adverse effects of A2E, efforts to retard A2E deposition in RPE cells could serve to combat visual loss in Stargardt's disease. Thus it is significant that in this issue of PNAS, Radu et al. (16) report that systemic drug therapy can reduce A2E accumulation in a mouse model of recessive Stargardt's disease (abcr−/−).
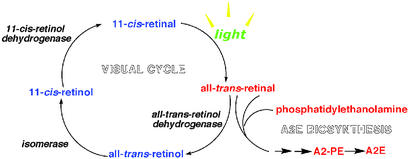
A2E was originally named from the two equivalents of vitamin A aldehyde (all-trans-retinal) and one equivalent of ethanolamine that are used as starting materials for its preparation (7). In the eye, A2E forms when an excess of all-trans-retinal leads to condensation reactions between all-trans-retinal and phosphatidylethanolamine (2:1 ratio) in photoreceptor outer segment disks (6, 17–19) (Fig. 1). To interfere with A2E synthesis, Radu et al. (16) treated mice with isotretinoin (13-cis-retinoic acid) to slow the visual cycle (20), the multistep pathway that is the source of all-trans-retinal for A2E production (Fig. 1). This approach followed earlier work showing that RPE lipofuscin can be profoundly decreased in animals lacking the 11-cis and all-trans-retinal chromophores caused either by dietary deficiency (21) or gene knockout (22). Light exposure, which drives the flux of all-trans-retinal through the visual cycle, had also been known to modulate A2E synthesis (18, 19).
Figure 1.
The visual cycle and A2E biosynthesis. All-trans-retinal that evades reduction to retinol can react with phosphatidylethanolamine (2:1 ratio) to generate A2E in a multistep process.
In the studies of Radu et al. (16), isotretinoin treatment retarded the formation of 11-cis-retinal, the photosensitive component of visual pigment that upon absorbing a photon undergoes isomerization to all-trans-retinal. Accordingly, both the WT and abcr null mutant mice exhibited isotretinoin-induced delays in dark adaptation, a property that is sensitive to levels of visual pigment (rhodopsin, in the case of rods). A similar delay in dark adaptation has been reported in mice carrying an amino acid substitution (leucine to methionine) at residue 450 of RPE-65 (23, 24), a protein that is essential to the regeneration of 11-cis-retinal. Because this allelic variation, as with isotretinoin treatment, leads to impeded 11-cis- retinal production, it may be an example of a gene variant that is associated with reduced A2E formation (22).
Of course, only a minute fraction of the all-trans-retinal that is released upon photoisomerization of 11-cis-retinal is available to form A2E. In what is the rate-determining step in the visual cycle, most all-trans-retinal is reduced to all-trans-retinol by the enzyme all-trans-retinol dehydrogenase located in the photoreceptor outer segment (25). Thus, only all-trans-retinal that escapes conversion can enter the A2E biosynthetic pathway. It follows that an additional strategy to limit the formation of A2E would be to facilitate the activity of all-trans-retinol dehydrogenase. Another option could involve intercepting the A2E biosynthetic pathway. Thus after sequential condensation reactions between phosphatidylethanolamine and all-trans-retinal, the synthesis of A2E proceeds through rearrangements and an autoxidative step, after which A2-PE, a fluorescent phosphatidyl-pyridinium bisretinoid is formed (6, 17–19). This pigment is the precursor of A2E that accumulates in photoreceptor outer segments (17, 19). Any of the intermediates formed before the oxidation step that generates A2-PE could likely undergo reversal. But because the oxidation completes the formation of the pyridinium ring and in doing so generates a stable compound the oxidation step is likely to be the last stage at which intervention may be possible.
This discussion has so far not accounted for the fact that the visual deficit in Stargardt's disease primarily involves the center of the field of vision. That RPE cells underlying the macula have the highest accumulations of lipofuscin (26, 27) goes a long way toward an explanation. But why should lipofuscin levels be greatest in the macula? From the perspective of the origins of RPE lipofuscin, most of the latter is retinoid-derived (22). This is certainly the case for A2E. Thus it is not a coincidence that the macula of the retina also has the highest concentration of 11-cis-retinal-containing visual pigment, a feature that reflects, in part, the packing density of cone and rod photoreceptor cells (28–30). The heightened capacity for photon absorption conferred by the density of visual pigment in the macula translates into a higher probability that all-trans-retinal will be available for A2E formation. Accordingly, greater amounts of A2E are amassed by RPE cells underlying the macula. Because lifetime accumulations of A2E are also greatest in the macula (3), critical levels of the fluorophore may be a factor in the onset of age-related macular degeneration.
Isotretinoin may be known to many readers as a medication (Accutane) used to treat severe acne. During treatment with Accutane, patients exhibit delayed dark adaptation, an abnormality that is manifested when an individual makes a transition from bright to dim light (31). However, this side effect is reversed when the drug is discontinued. An important aspect of studies that examined isotretinoin administration in rats (20) and mice (16) was that, despite delayed dark adaptation, photoreceptor cell loss did not occur and visual function was normal in mice that had been dark-adapted. For the correction of A2E accumulation, Radu et al. (16) pointed out that it may be possible to achieve therapeutic levels of isotretinoin by administering daily doses that are comparable to that prescribed for acne. Nonetheless, it should be noted that when administered orally isotretinoin is well known to cause birth defects (32). For a course of therapy, which is typically 3–4 months to treat acne, female patients must adhere to a strict protocol to prevent pregnancy. This issue and the significance of other side effects (33) have to be resolved if long-term isotretinoin therapy for Stargardt's disease is contemplated. The importance of these considerations notwithstanding, the efficacy with which isotretinoin reduced A2E accumulation in an experimental model (16) suggests a therapeutic strategy that eventually could involve isotretinoin or a synthetic analog in the treatment of Stargardt's macular degeneration. Until then, a safe and inexpensive approach is to reduce A2E accumulation by avoiding the bright light conditions that accelerate the flux of all-trans-retinal. If, in addition to reducing overall light exposure, lenses (yellow filters) that limit the amount of blue light reaching the RPE are chosen protection against A2E photoreactivity may also be gained.
Footnotes
See companion article on page 4742.
References
- 1.Sun H, Nathans J. Sci Am. 2001;285(4):68–75. doi: 10.1038/scientificamerican1001-68. [DOI] [PubMed] [Google Scholar]
- 2.Lopez P F, Maumenee I H, de la Cruz Z, Green W R. Ophthalmology. 1990;97:798–809. doi: 10.1016/s0161-6420(90)32508-3. [DOI] [PubMed] [Google Scholar]
- 3.Delori F C, Goger D G, Dorey C K. Invest Ophthalmol Visual Sci. 2001;42:1855–1866. [PubMed] [Google Scholar]
- 4.Eldred G E, Katz M L. Exp Eye Res. 1988;47:71–86. doi: 10.1016/0014-4835(88)90025-5. [DOI] [PubMed] [Google Scholar]
- 5.Eldred G E, Lasky M R. Nature. 1993;361:724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- 6.Parish C A, Hashimoto M, Nakanishi K, Dillon J, Sparrow J R. Proc Natl Acad Sci USA. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakai N, Decatur J, Nakanishi K, Eldred G E. J Am Chem Soc. 1996;118:1559–1560. [Google Scholar]
- 8.Sparrow J R, Parish C A, Hashimoto M, Nakanishi K. Invest Ophthalmol Visual Sci. 1999;40:2988–2995. [PubMed] [Google Scholar]
- 9.De S, Sakmar T P. J Gen Physiol. 2002;120:147–157. doi: 10.1085/jgp.20028566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holz F G, Schutt F, Kopitz J, Eldred G E, Kruse F E, Volcker H E, Cantz M. Invest Ophthalmol Visual Sci. 1999;40:737–743. [PubMed] [Google Scholar]
- 11.Finneman S C, Leung L W, Rodriguez-Boulan E. Proc Natl Acad Sci USA. 2002;99:3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suter M, Reme C E, Grimm C, Wenzel A, Jaattela M, Esser P, Kociok N, Leist M, Richter C. J Biol Chem. 2000;275:39625–39630. doi: 10.1074/jbc.M007049200. [DOI] [PubMed] [Google Scholar]
- 13.Sparrow J R, Nakanishi K, Parish C A. Invest Ophthalmol Visual Sci. 2000;41:1981–1989. [PubMed] [Google Scholar]
- 14.Sparrow J R, Cai B. Invest Ophthalmol Visual Sci. 2001;42:1356–1362. [PubMed] [Google Scholar]
- 15.Sparrow J R, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Invest Ophthalmol Visual Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- 16.Radu R A, Mata N L, Nusinowitz S, Liu X, Sieving P A, Travis G H. Proc Natl Acad Sci USA. 2003;100:4742–4747. doi: 10.1073/pnas.0737855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow J R. J Biol Chem. 2000;275:29354–29360. doi: 10.1074/jbc.M910191199. [DOI] [PubMed] [Google Scholar]
- 18.Mata N L, Weng J, Travis G H. Proc Natl Acad Sci USA. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Shabat S, Parish C A, Vollmer H R, Itagaki Y, Fishkin N, Nakanishi K, Sparrow J R. J Biol Chem. 2002;277:7183–7190. doi: 10.1074/jbc.M108981200. [DOI] [PubMed] [Google Scholar]
- 20.Sieving P A, Chaudhry P, Kondo M, Provenzano M, Wu D, Carlson T J, Bush R A, Thompson D A. Proc Natl Acad Sci USA. 2001;98:1835–1840. doi: 10.1073/pnas.041606498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz M L, Norberg M, Stientjes H J. Invest Ophthalmol Visual Sci. 1992;33:2612–2618. [PubMed] [Google Scholar]
- 22.Katz M L, Redmond T M. Invest Ophthalmol Visual Sci. 2001;42:3023–3030. [PubMed] [Google Scholar]
- 23.Wenzel A, Reme C E, Williams T P, Hafezi F, Grimm C. J Neurosci. 2001;21:53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danciger M, Matthes M T, Yasamura D, Akhmedov N B, Rickabaugh T, Gentleman S, Redmond T M, La Vail M M, Farber D B. Mamm Genome. 2000;11:422–427. doi: 10.1007/s003350010081. [DOI] [PubMed] [Google Scholar]
- 25.McBee J K, Palczewski K, Baehr W, Pepperberg D R. Prog Retinal Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 26.Lois N, Holder G E, Fitzke F W, Plant C, Bird A C. Invest Ophthalmol Visual Sci. 1999;40:2668–2675. [PubMed] [Google Scholar]
- 27.Eagle R C, Lucier A C, Bernardino V B, Yanoff M. Ophthalmology. 1980;87:1189–1200. doi: 10.1016/s0161-6420(80)35106-3. [DOI] [PubMed] [Google Scholar]
- 28.Faulkner D J, Kemp C M. Vision Res. 1984;24:221–231. doi: 10.1016/0042-6989(84)90124-x. [DOI] [PubMed] [Google Scholar]
- 29.Liem A T A, Keunen J E E, Van Norren D. Surv Ophthalmol. 1996;41:37–50. doi: 10.1016/s0039-6257(97)81994-7. [DOI] [PubMed] [Google Scholar]
- 30.Tournow R P, Stilling R. Acta Anat. 1998;162:163–168. doi: 10.1159/000046482. [DOI] [PubMed] [Google Scholar]
- 31.Weleber R G, Denman S T, Hanifin J M, Cunningham W J. Arch Ophthalmol. 1986;104:831–837. doi: 10.1001/archopht.1986.01050180065031. [DOI] [PubMed] [Google Scholar]
- 32.Ellis C N, Krach K J. J Am Acad Dermatol. 2001;45:S150–S157. doi: 10.1067/mjd.2001.113717. [DOI] [PubMed] [Google Scholar]
- 33.Di Giovanna J J. J Am Acad Dermatol. 2001;45:S176–S182. doi: 10.1067/mjd.2001.113721. [DOI] [PubMed] [Google Scholar]