Abstract
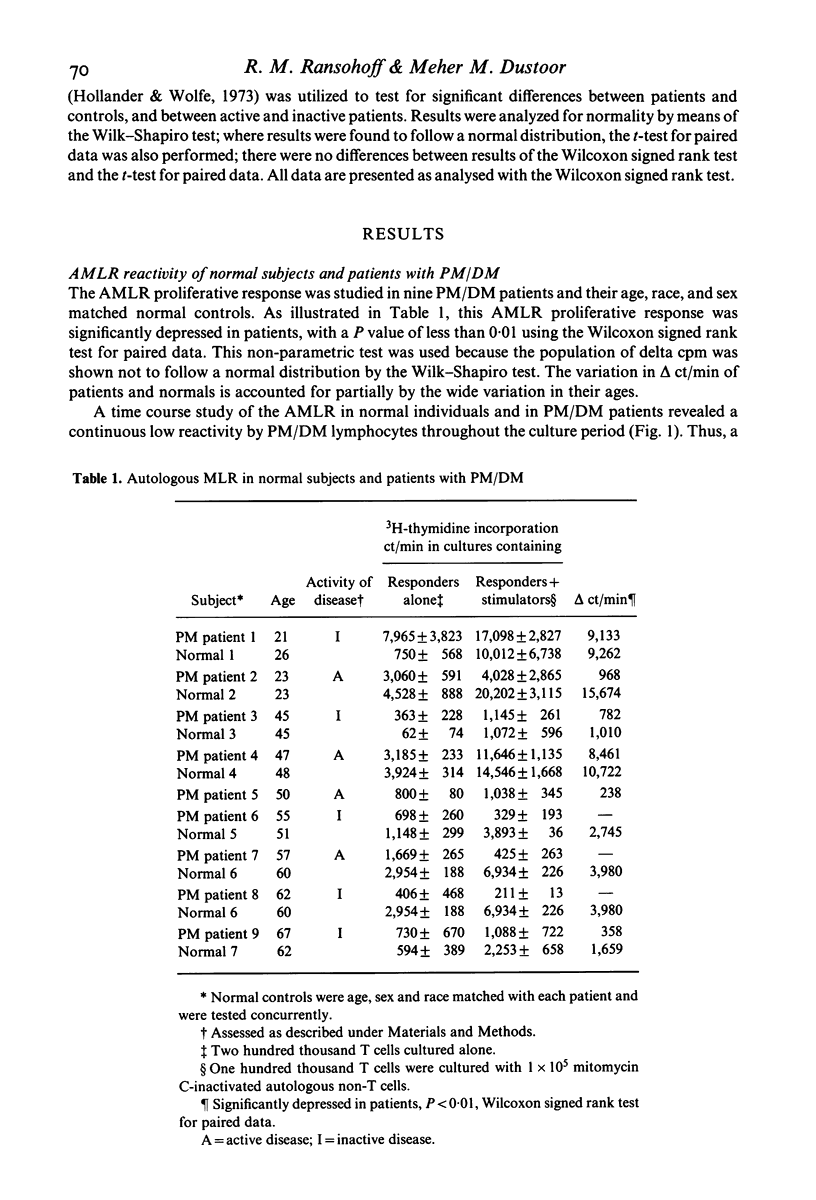
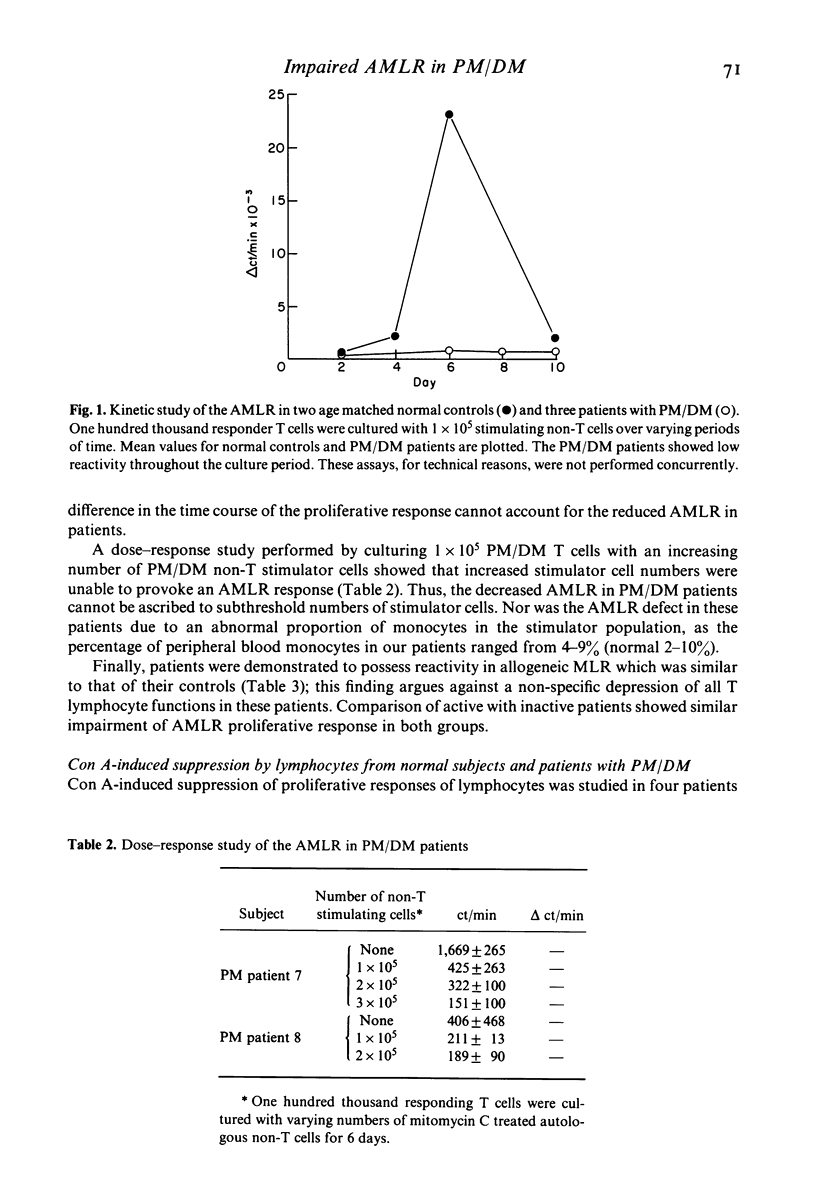
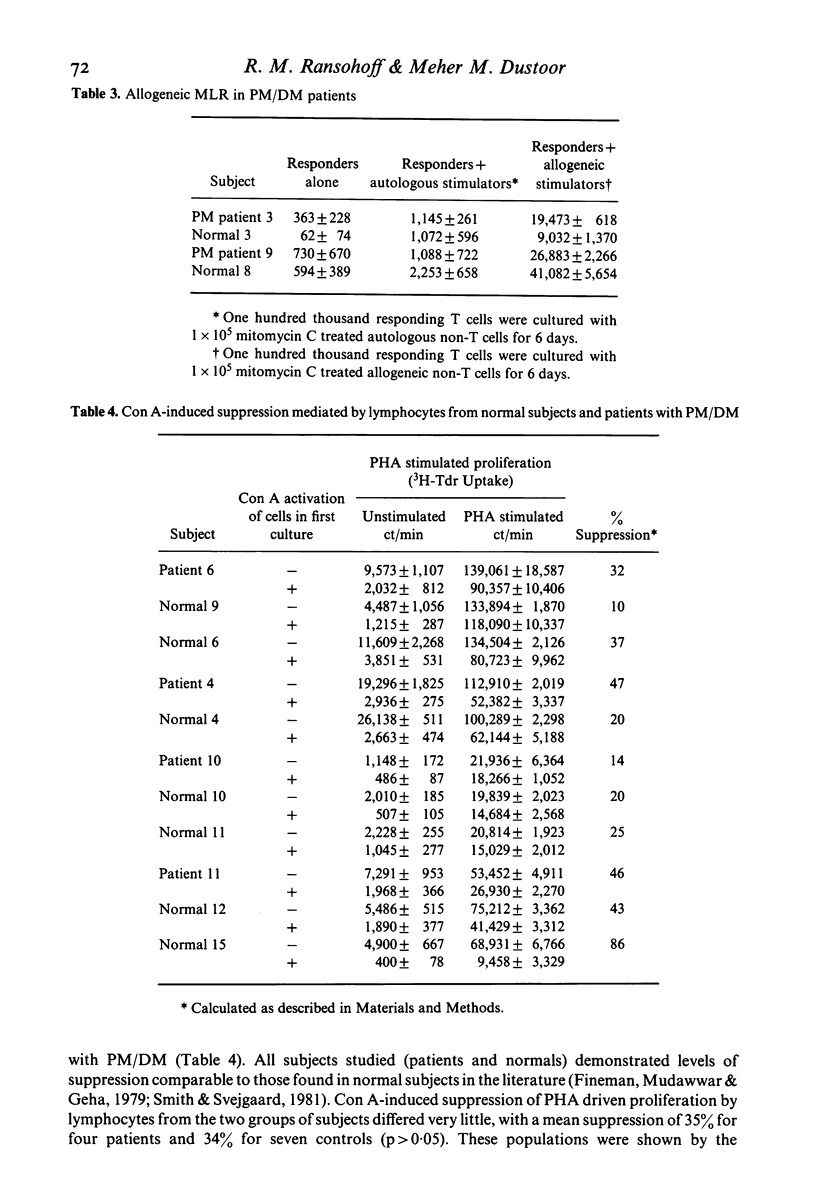
Polymyositis/dermatomyositis (PM/DM) is an autoimmune disorder of unknown aetiology. In order to study whether immunoregulatory abnormalities might be involved in this autoimmune state, we investigated the autologous mixed lymphocyte reaction (AMLR) and concanavalin A-induced suppressor cell function (Con A-induced suppression) in adult patients with primary PM/DM. We found the AMLR to be significantly depressed in patients; responsiveness could not be enhanced by increasing the numbers of non-T stimulator cells in culture, nor by varying the day on which cultures were harvested. Con A-induced suppression of T cell proliferative responses to mitogenic stimuli was normal. These findings implicate abnormal immunoregulation in the pathophysiology of PM/DM. Further, the dissociation of AMLR reactivity from Con A-inducible suppression suggests that events important for immunoregulatory competence may occur in the AMLR culture, despite the absence of an observed proliferative response.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battisto J. R., Ponzio N. M. Autologous and syngeneic mixed lymphocyte reactions and their immunological significance. Prog Allergy. 1981;28:160–192. [PubMed] [Google Scholar]
- Bohan A., Peter J. B., Bowman R. L., Pearson C. M. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore) 1977 Jul;56(4):255–286. doi: 10.1097/00005792-197707000-00001. [DOI] [PubMed] [Google Scholar]
- Carpenter S., Karpati G., Heller I., Eisen A. Inclusion body myositis: a distinct variety of idiopathic inflammatory myopathy. Neurology. 1978 Jan;28(1):8–17. doi: 10.1212/wnl.28.1.8. [DOI] [PubMed] [Google Scholar]
- Dawkins R. L. Experimental autoallergic myositis, polymyositis and myasthenia gravis. Autoimmune muscle disease associated with immunodeficiency and neoplasia. Clin Exp Immunol. 1975 Aug;21(2):185–201. [PMC free article] [PubMed] [Google Scholar]
- Dawkins R. L., Mastaglia F. L. Cell-mediated cytotoxicity to muscle in polymyositis. Effect of immunosuppression. N Engl J Med. 1973 Mar 1;288(9):434–438. doi: 10.1056/NEJM197303012880903. [DOI] [PubMed] [Google Scholar]
- Esiri M. M., MacLennan I. C., Hazleman B. L. Lymphocyte sensitivity to skeletal muscle in patients with polymyositis and other disorders. Clin Exp Immunol. 1973 May;14(1):25–35. [PMC free article] [PubMed] [Google Scholar]
- Fineman S. M., Mudawwar F. B., Geha R. S. Characteristics and mechanisms of action of the concanavalin A-activated suppressor cell in man. Cell Immunol. 1979 Jun;45(1):120–132. doi: 10.1016/0008-8749(79)90367-8. [DOI] [PubMed] [Google Scholar]
- Glimcher L. H., Shevach E. M. Production of autoreactive I region-restricted T cell hybridomas. J Exp Med. 1982 Aug 1;156(2):640–645. doi: 10.1084/jem.156.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D. C., Arnason B. G. Cell-mediated immunity in polymyositis. Creatine phosphokinase release from muscle cultures. Arch Neurol. 1974 Sep;31(3):192–196. doi: 10.1001/archneur.1974.00490390074008. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., MacDermott R. P., Jacobs S. B., Pletscher L. S., Beale M. G. Immunosuppressive effects of low doses of glucocorticoids: effects on autologous and allogeneic mixed leukocyte reactions. J Immunol. 1980 Jun;124(6):2812–2817. [PubMed] [Google Scholar]
- Hellmann D., Stobo J. The autologous mixed lymphocyte reaction. A precautionary note. Arthritis Rheum. 1982 Feb;25(2):121–125. doi: 10.1002/art.1780250201. [DOI] [PubMed] [Google Scholar]
- Huber C., Merkenschlager M., Gattringer C., Royston I., Fink U., Braunsteiner H. Human autologous mixed lymphocyte reactivity is primarily specific for xenoprotein determinants adsorbed to antigen-presenting cells during rosette formation with sheep erythrocytes. J Exp Med. 1982 Apr 1;155(4):1222–1227. doi: 10.1084/jem.155.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes J. B., Kuntz M. M., Kim Y. T., Weksler M. E. Induction of suppressor activity in the autologous mixed lymphocyte reaction and in cultures with concanavalin A. J Clin Invest. 1979 Dec;64(6):1608–1613. doi: 10.1172/JCI109622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. L., Fink C. W., Ziff M. Lymphotoxin formation by lymphocytes and muscle in polymyositis. J Clin Invest. 1972 Sep;51(9):2435–2449. doi: 10.1172/JCI107057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakulas B. A. Destruction of differentiated muscle cultures by sensitised lymphoid cells. J Pathol Bacteriol. 1966 Apr;91(2):495–503. doi: 10.1002/path.1700910225. [DOI] [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. Lymphocyte transformation induced by autologous cells. IV. Human T-lymphocyte proliferation induced by autologous or allogeneic non-T lymphocytes. J Exp Med. 1976 May 1;143(5):1042–1054. doi: 10.1084/jem.143.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Stacey M. C. Further characterization of the human autologous mixed leukocyte reaction (MLR). J Immunol. 1981 Feb;126(2):729–734. [PubMed] [Google Scholar]
- Miyasaka N., Sauvezie B., Pierce D. A., Daniels T. E., Talal N. Decreased autologous mixed lymphocyte reaction in Sjögren's syndrome. J Clin Invest. 1980 Nov;66(5):928–933. doi: 10.1172/JCI109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody C. E., Casazza B. A., Christenson W. N., Weksler M. E. Lymphocyte transformation induced by autologous cells. VIII. Impaired autologous mixed lymphocyte reactivity in patients with acute infectious mononucleosis. J Exp Med. 1979 Dec 1;150(6):1448–1455. doi: 10.1084/jem.150.6.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge T. A., Smith P. D. A quantitative test to detect lymphocytes sensitized against the surface of muscle cells. Clin Exp Immunol. 1976 Jul;25(1):139–143. [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Sakane T., Green I. Specificity and suppressor function of human T cells responsive to autologous non-T cells. J Immunol. 1979 Aug;123(2):584–589. [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Failure of autologous mixed lymphocyte reactions between T and non-T cells in patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3464–3468. doi: 10.1073/pnas.75.7.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum. 1978 Jul-Aug;21(6):657–664. doi: 10.1002/art.1780210608. [DOI] [PubMed] [Google Scholar]
- Shevach E. M. The function of macrophages in antigen recognition by guinea pig T lymphocytes. III. Genetic analysis of the antigens mediating macrophage-T lymphocyte interaction. J Immunol. 1976 May;116(5):1482–1489. [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. I., Svejgaard A. Concanavalin-A-induced suppressor lymphocytes in normal individuals. Scand J Immunol. 1981;13(5):483–492. doi: 10.1111/j.1365-3083.1981.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Talal N. Significance of self-recognition and interleukin-2 for immunoregulation, autoimmunity and cancer. Scand J Immunol. 1982 Oct;16(4):269–278. doi: 10.1111/j.1365-3083.1982.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Smolen J. S., Chused T. M., Novotny E. A., Steinberg A. D. The human autologous mixed lymphocyte reaction. III. Immune circuits. J Immunol. 1982 Sep;129(3):1050–1053. [PubMed] [Google Scholar]
- Weksler M. E., Kozak R. Lymphocyte transformation induced by autologous cells. V. Generation of immunologic memory and specificity during the autologous mixed lymphocyte reaction. J Exp Med. 1977 Dec 1;146(6):1833–1838. doi: 10.1084/jem.146.6.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler M. E., Moody C. E., Jr, Kozak R. W. The autologous mixed-lymphocyte reaction. Adv Immunol. 1981;31:271–312. doi: 10.1016/s0065-2776(08)60923-2. [DOI] [PubMed] [Google Scholar]
- Ziff M., Johnson R. L. Polymyositis and cell-mediated immunity. N Engl J Med. 1973 Mar 1;288(9):465–466. doi: 10.1056/NEJM197303012880910. [DOI] [PubMed] [Google Scholar]



