Abstract
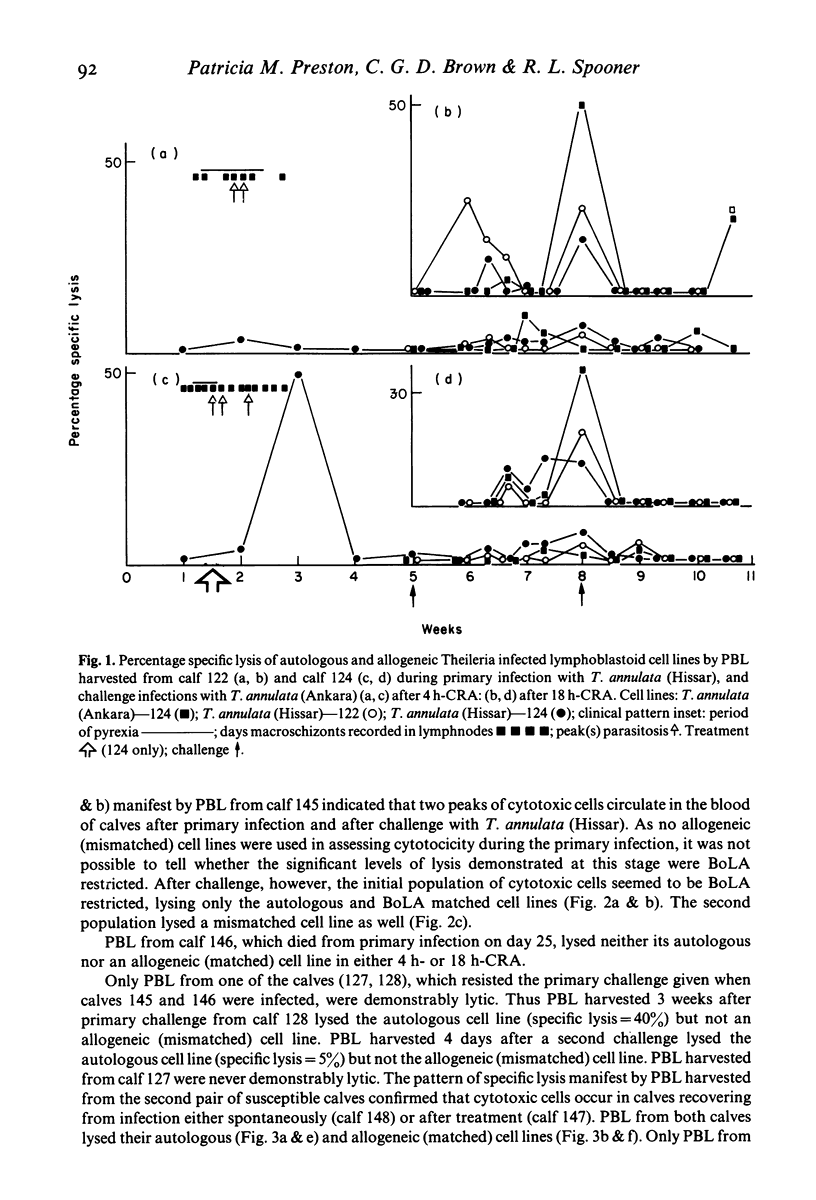
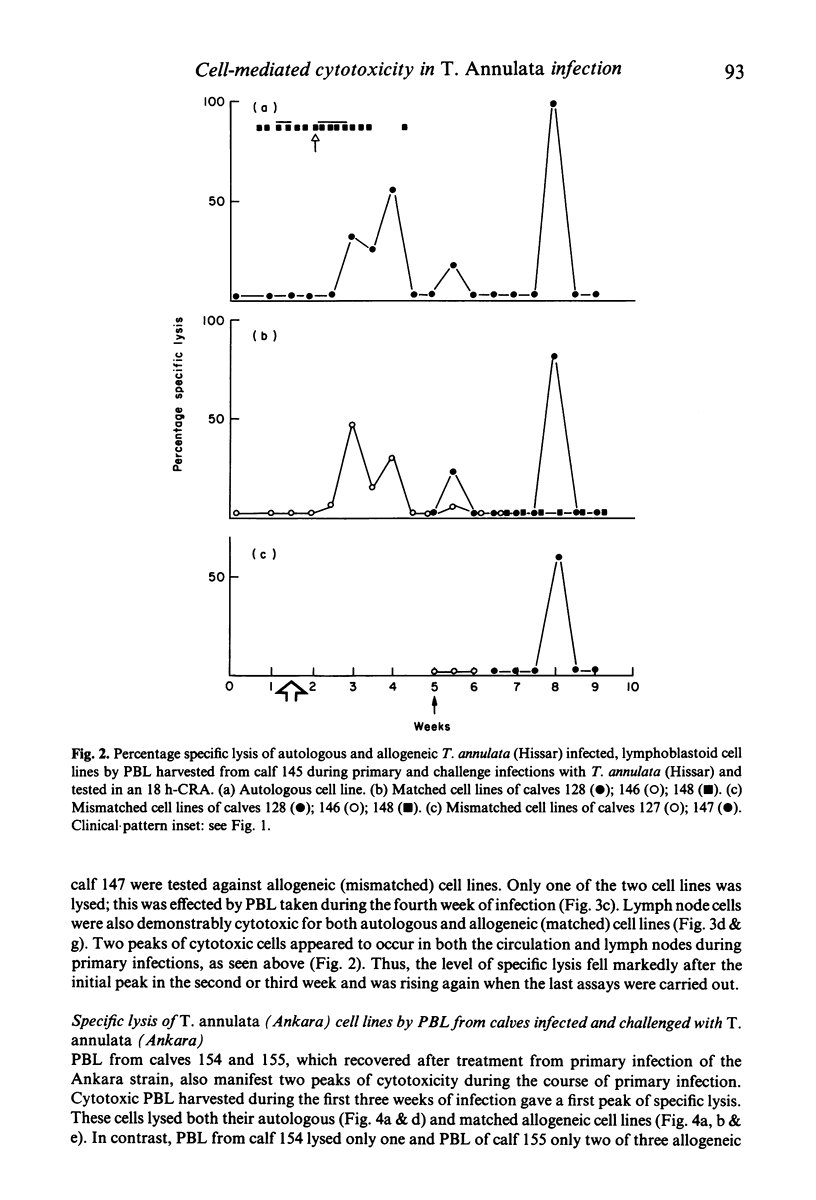
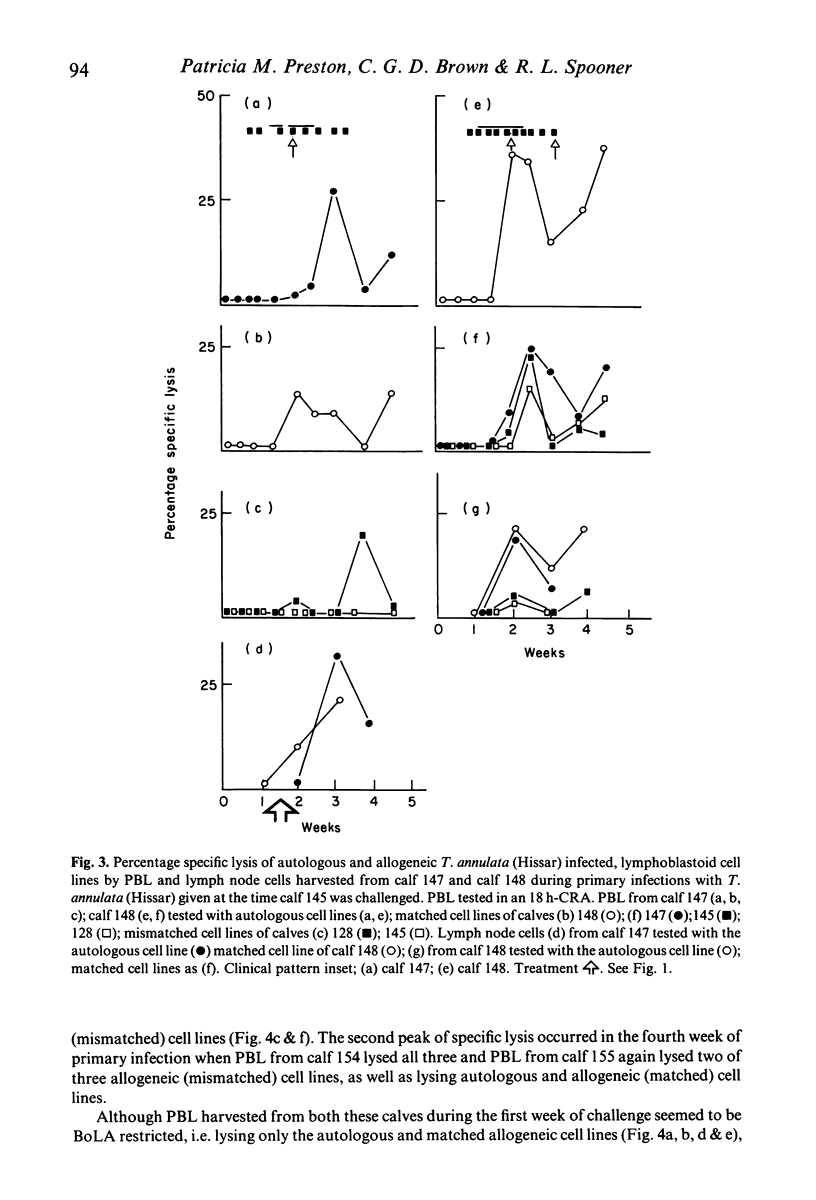
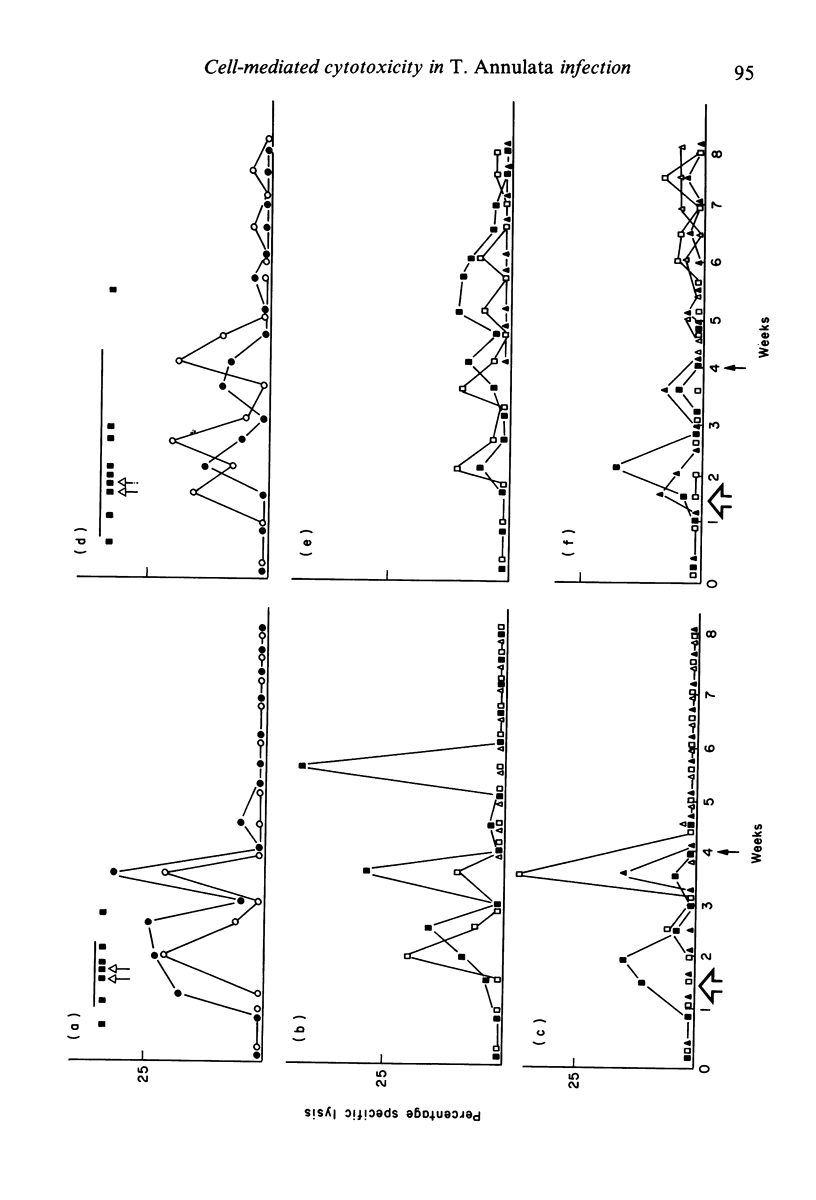
Recovery of calves from tropical theileriosis was accompanied by the disappearance of macroschizonts from lymph nodes and the appearance of cytotoxic cells in the blood and lymph nodes. Acute, fatal disease was associated with incremental parasitosis and parasitaemia and, in general, an absence of detectable cytotoxic cells in the blood or lymph nodes. After recovery from infection, calves were resistant to challenge. Challenge with sporozoites was followed sometimes by an immediate reappearance or by a later peak, or sometimes by twin peaks of cytotoxic cells but macroschizonts were not detected. Histocompatibility (BoLA) typing indicated that calves produced two sequential populations of cytotoxic cells during recovery from primary infection with Theileria annulata. The expression of lysis by the first appeared to be BoLA restricted. In contrast, both the peaks of lysis manifest after challenge appeared to be BoLA restricted. Results suggest that BoLA restricted cells are established in the immunological memory and are probably analogous to cytotoxic T cells, while non-BoLA restricted cytotoxic cells are natural killer like cells. The results suggest a role for cytotoxic cells in recovery from primary infection, in the inhibition of proliferation of macroschizonts which evade mechanisms of acquired resistance and in the lysis of macroschizont infected cells deriving from challenge sporozoites which have evaded serum-mediated inhibition.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emery D. L., Eugui E. M., Nelson R. T., Tenywa T. Cell-mediated immune responses to Theileria parva (East Coast fever) during immunization and lethal infections in cattle. Immunology. 1981 Jun;43(2):323–336. [PMC free article] [PubMed] [Google Scholar]
- Eugui E. M., Emery D. L. Genetically restricted cell-mediated cytotoxicity in cattle immune to Theileria parva. Nature. 1981 Mar 19;290(5803):251–254. doi: 10.1038/290251a0. [DOI] [PubMed] [Google Scholar]
- Gill B. S., Bhattacharyulu Y., Kaur D. Immunisation against bovine tropical theileriasis (Theileria annulata infection). Res Vet Sci. 1976 Sep;21(2):146–149. [PubMed] [Google Scholar]
- Klein G., Purtilo D. Summary: symposium on Epstein-Barr virus-induced lymphoproliferative diseases in immunodeficient patients. Cancer Res. 1981 Nov;41(11 Pt 1):4302–4304. [PubMed] [Google Scholar]
- Moss D. J., Wallace L. E., Rickinson A. B., Epstein M. A. Cytotoxic T cell recognition of Epstein-Barr virus-infected B cells. I. Specificity and HLA restriction of effector cells reactivated in vitro. Eur J Immunol. 1981 Sep;11(9):686–693. doi: 10.1002/eji.1830110904. [DOI] [PubMed] [Google Scholar]
- Musoke A. J., Nantulya V. M., Buscher G., Masake R. A., Otim B. Bovine immune response to Theileria parva: neutralizing antibodies to sporozoites. Immunology. 1982 Apr;45(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Oliver R. A., McCoubrey C. M., Millar P., Morgan A. L., Spooner R. L. A genetic study of bovine lymphocyte antigens (BoLA) and their frequency in several breeds. Immunogenetics. 1981;13(1-2):127–132. doi: 10.1007/BF00524610. [DOI] [PubMed] [Google Scholar]
- Pearson T. W., Hewett R. S., Roelants G. E., Stagg D. A., Dolan T. T. Studies on the induction and specificity of cytotoxicity to Theileria-transformed cell lines. J Immunol. 1982 Jun;128(6):2509–2513. [PubMed] [Google Scholar]
- Pearson T. W., Lundin L. B., Dolan T. T., Stagg D. A. Cell-mediated immunity to Theileria-transformed cell lines. Nature. 1979 Oct 25;281(5733):678–680. doi: 10.1038/281678a0. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Wallace L. E., Epstein M. A. HLA-restricted T-cell recognition of Epstein-Barr virus-infected B cells. Nature. 1980 Feb 28;283(5750):865–867. doi: 10.1038/283865a0. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. The direct antiviral cytotoxicity by bovine lymphocytes is not restricted by genetic incompatibility of lymphocytes and target cells. J Immunol. 1977 Feb;118(2):618–624. [PubMed] [Google Scholar]
- Schein E. On the life cycle of Theileria annulata (Dschunkowsky and Luhs, 1904) in the midgut and hemolymph of Hyalomma anatolicum excavatum (Koch, 1844). Z Parasitenkd. 1975 Sep 12;47(2):165–167. doi: 10.1007/BF00382639. [DOI] [PubMed] [Google Scholar]
- Spooner R. L., Brown C. G. Bovine lymphocyte antigens (BoLA) of bovine lymphocytes and derived lymphoblastoid lines transformed by Theileria parva and Theileria annulata. Parasite Immunol. 1980 Autumn;2(3):163–174. doi: 10.1111/j.1365-3024.1980.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]



