Abstract
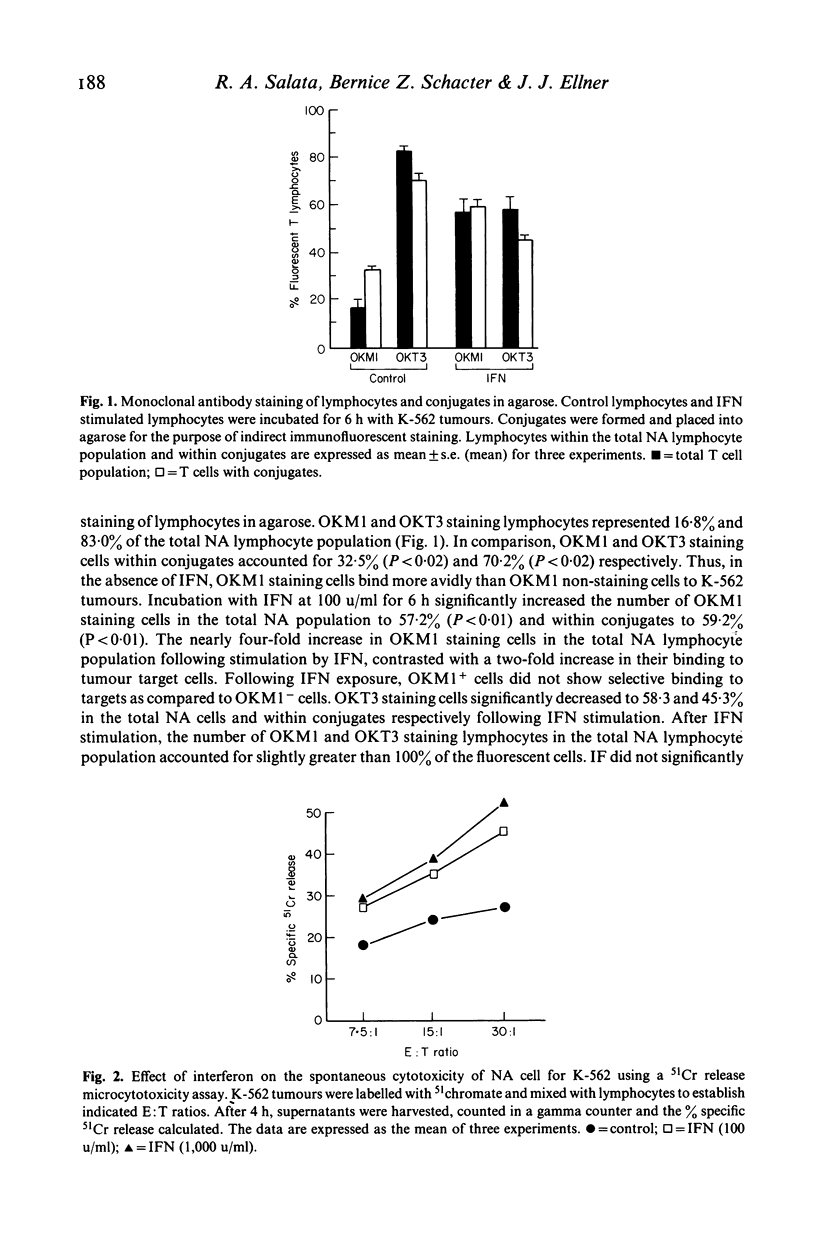
Spontaneous cytotoxicity of human lymphocytes for tumours is increased by interferon (IFN) without change in the overall fraction of cells binding to targets. We developed an indirect immunofluorescent technique to stain lymphocytes conjugated to K-562 tumour cells in agarose with monoclonal antibodies. This allowed assessment of lymphocyte subpopulations binding to tumour cells without disruption of conjugates. Overall binding of non-adherent (NA) lymphocytes to tumour targets following incubation at 37 degrees C for 6 h was 13.3 +/- 0.3% compared to 12.5 +/- 0.7% with inclusion of IFN at 100 u/ml. When NA lymphocytes were incubated with K-562 tumour cells without IFN, OKM1 and OKT3 staining lymphocytes comprised 16.8 +/- 3.5% and 83.0 +/- 1.3% of the total lymphocyte population and 32.5 +/- 1.3% and 70.2 +/- 2.6% of lymphocytes conjugated to tumours. Incubation with IFN significantly increased OKM1 staining cells in the total NA population to 57.2 +/- 5.6% (P less than 0.01) and within tumour conjugates to 59.2 +/- 2.7% (P less than 0.01) while OKT3 staining cells decreased to 58.3 +/- 5.2% (P less than 0.02) and 45.3 +/- 1.2% (P less than 0.01), respectively. IFN increased cytotoxicity of NA cells for 51Cr-labelled K-562 by 66% at an effector to target ratio of 30:1 (P less than 0.001). These results demonstrate that OKM1 staining cells bind more avidly to tumour targets in the absence of IFN. IFN selectively increases the proportion of OKM1 staining lymphocytes with a concomitant increase in their binding to tumour cells. Therefore, enhancement of cytotoxicity by IFN in the NK system may result, in part, from conversion of OKT3 to OKM1 staining cells which are more efficient killers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R. Interferons and the immune system. Nature. 1980 Apr 17;284(5757):593–595. doi: 10.1038/284593a0. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Grimm E., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. I. Estimation of cytotoxic T lymphocyte frequency and relative lytic efficiency. J Immunol. 1979 Dec;123(6):2861–2869. [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Jondal M., Spine C., Targan S. Human spontaneous killer cells selective for tumour-derived target cells. Nature. 1978 Mar 2;272(5648):62–64. doi: 10.1038/272062a0. [DOI] [PubMed] [Google Scholar]
- Kay H. D., Horwitz D. A. Evidence by reactivity with hybridoma antibodies for a probable myeloid origin of peripheral blood cells active in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Clin Invest. 1980 Oct;66(4):847–851. doi: 10.1172/JCI109923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Moretta L., Roper M., Breard J. M., Mingari M. C., Cooper M. D., Schlossman S. F. Human T lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. A comparison. J Exp Med. 1980 Apr 1;151(4):969–974. doi: 10.1084/jem.151.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Rubin P., Pross H. F., Roder J. C. Studies of human natural killer cells. II. Analysis at the single cell level. J Immunol. 1982 Jun;128(6):2553–2558. [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Lief F. S. Cell-mediated cytotoxicity against virus-infected target cells in humans. I. Characterization of the effector lymphocyte. J Immunol. 1978 Aug;121(2):526–531. [PubMed] [Google Scholar]
- Silva A., Bonavida B., Targan S. Mode of action of interferon-mediated modulation of natural killer cytotoxic activity: recruitment of pre-NK cells and enhanced kinetics of lysis. J Immunol. 1980 Aug;125(2):479–484. [PubMed] [Google Scholar]
- Targan S., Dorey F. Interferon activation of "pre-spontaneous killer" (pre-SK) cells and alteration in kinetics of lysis of both "pre-SK" and active SK cells. J Immunol. 1980 May;124(5):2157–2161. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Analysis by a single cell cytotoxicity assay of natural killer (NK) cells frequencies among human large granular lymphocytes and of the effects of interferon on their activity. J Immunol. 1982 Jun;128(6):2514–2521. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West W. H., Cannon G. B., Kay H. D., Bonnard G. D., Herberman R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977 Jan;118(1):355–361. [PubMed] [Google Scholar]
- Zarling J. M., Kung P. C. Monoclonal antibodies which distinguish between human NK cells and cytotoxic T lymphocytes. Nature. 1980 Nov 27;288(5789):394–396. doi: 10.1038/288394a0. [DOI] [PubMed] [Google Scholar]
- van de Griend R. J., ten Berge I., Tanke H. J., Roos D., Schellekens P. T., Melief C. J., Zeijlemaker W. P., Astaldi A. Characterization of two subsets of human T gamma cells. J Immunol. 1982 May;128(5):1979–1985. [PubMed] [Google Scholar]


