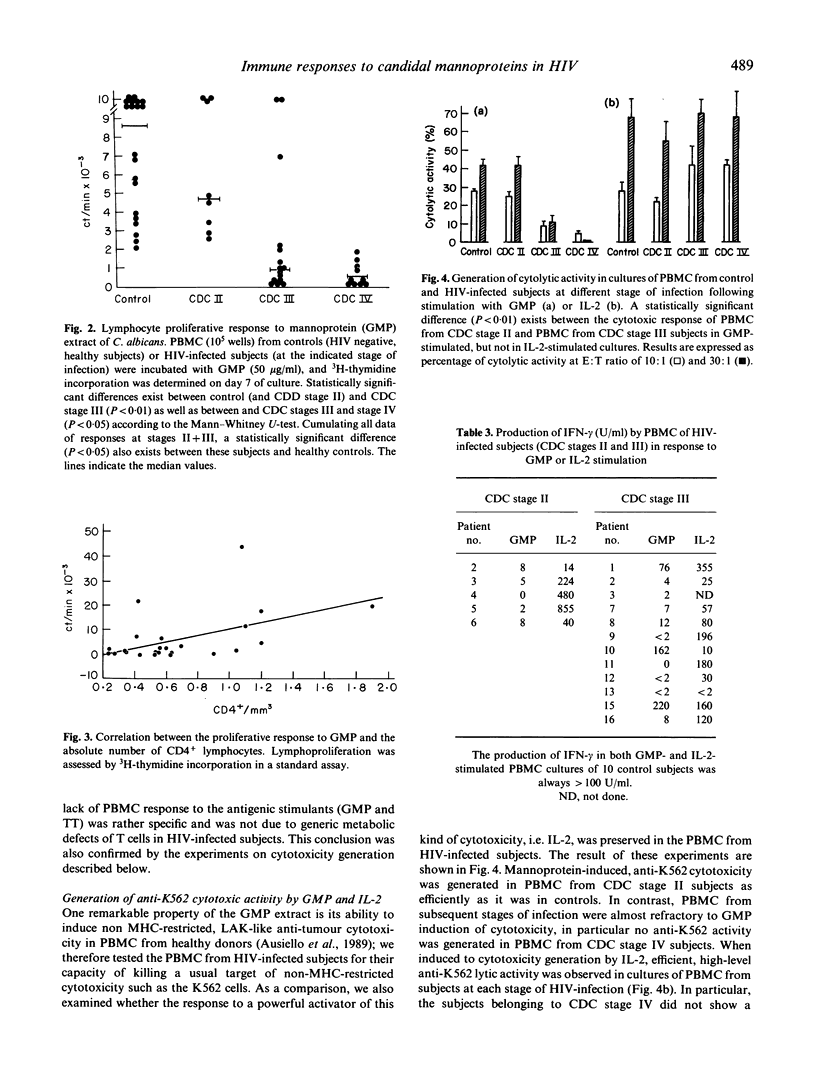
Abstract
Mucosal candidiasis is one of the first opportunistic diseases in HIV-infected subjects. In order to understand the relationship between this disease and immunodeficiency to chemically defined, immunodominant Candida antigens, a mannoprotein fraction from C. albicans cell wall (GMP) was used to analyse proliferative and non-MHC-restricted cytotoxic responses of peripheral blood mononuclear cells (PBMC) from normal and HIV-infected subjects. In the former, GMP induced extensive blastogenesis, generation of powerful cytotoxicity against a tumour cell line (K562), and production of substantial amounts of interferon-gamma (IFN-gamma). Cultured PBMC from HIV-infected subjects manifested an early decreased ability for proliferative as well as differentiative cytotoxic responses to the candidal mannoproteins. This inability became clearly evident in subjects with stage III (CDC) of the disease, was total in CDC stage IV and occurred even in some subjects with a normal number of CD4+ cells. Low or absent response to GMP correlated with lack of response to tetanus toxoid. In contrast, both lymphoproliferative and cytotoxic responses to exogenous IL-2 was highly preserved at all stages of infection. The production of IFN-gamma in GMP-stimulated PBMC cultures critically fell to negligible values in most of the subjects in CDC stages II and III. Thus, the lowered or absent cell-mediated immune responses to candidal mannoprotein may be one factor to explain the early, elevated susceptibility of HIV-infected subjects to mucosal candidiasis. This study also shows that our mannoprotein preparation may be used as a probe to detect the overall efficiency of T cell responses in the above subjects.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausiello C. M., Palma C., Spagnoli G. C., Piazza A., Casciani C. U., Cassone A. Cytotoxic effectors in human peripheral blood mononuclear cells induced by a mannoprotein complex of Candida albicans: a comparison with interleukin 2-activated killer cells. Cell Immunol. 1989 Jul;121(2):349–359. doi: 10.1016/0008-8749(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Oral Candida albicans isolates from nonhospitalized normal carriers, immunocompetent hospitalized patients, and immunocompromised patients with or without acquired immunodeficiency syndrome. J Clin Microbiol. 1989 Jun;27(6):1335–1341. doi: 10.1128/jcm.27.6.1335-1341.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow E. W., Domer J. E. Immunoregulation in experimental murine candidiasis: specific suppression induced by Candida albicans cell wall glycoprotein. Infect Immun. 1985 Jul;49(1):172–181. doi: 10.1128/iai.49.1.172-181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A., Torosantucci A., Boccanera M., Pellegrini G., Palma C., Malavasi F. Production and characterisation of a monoclonal antibody to a cell-surface, glucomannoprotein constituent of Candida albicans and other pathogenic Candida species. J Med Microbiol. 1988 Dec;27(4):233–238. doi: 10.1099/00222615-27-4-233. [DOI] [PubMed] [Google Scholar]
- Cuff C. F., Packer B. J., Rogers T. J. A further characterization of Candida albicans-induced suppressor B-cell activity. Immunology. 1989 Sep;68(1):80–86. [PMC free article] [PubMed] [Google Scholar]
- Domer J. E., Stashak P. W., Elkins K., Prescott B., Caldes G., Baker P. J. Separation of immunomodulatory effects of mannan from Candida albicans into stimulatory and suppressive components. Cell Immunol. 1986 Sep;101(2):403–414. doi: 10.1016/0008-8749(86)90153-x. [DOI] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Le Deist F., Drouhet E., Griscelli C. Mannan-specific and mannan-induced T-cell suppressive activity in patients with chronic mucocutaneous candidiasis. J Clin Immunol. 1987 Sep;7(5):400–409. doi: 10.1007/BF00917018. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fontana L., Sirianni M. C., de Sanctis G., Carbonari M., Ensoli B., Aiuti F. Deficiency of natural killer activity, but not of natural killer binding, in patients with lymphoadenopathy syndrome positive for antibodies to HTLV-III. Immunobiology. 1986 Jul;171(4-5):425–435. doi: 10.1016/S0171-2985(86)80074-2. [DOI] [PubMed] [Google Scholar]
- Gruters R. A., Terpstra F. G., De Jong R., Van Noesel C. J., Van Lier R. A., Miedema F. Selective loss of T cell functions in different stages of HIV infection. Early loss of anti-CD3-induced T cell proliferation followed by decreased anti-CD3-induced cytotoxic T lymphocyte generation in AIDS-related complex and AIDS. Eur J Immunol. 1990 May;20(5):1039–1044. doi: 10.1002/eji.1830200514. [DOI] [PubMed] [Google Scholar]
- Heagy W., Strom T. B., Kelley V. E., Collela J., Crumpacker C., Williams J. M., Shapiro H. M., Laubenstein L., Finberg R. Recombinant human gamma interferon enhances in vitro activation of lymphocytes isolated from patients with acquired immunodeficiency syndrome. Infect Immun. 1989 Nov;57(11):3619–3628. doi: 10.1128/iai.57.11.3619-3628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann B., Jakobsen K. D., Odum N., Dickmeiss E., Platz P., Ryder L. P., Pedersen C., Mathiesen L., Bygbjerg I. B., Faber V. HIV-induced immunodeficiency. Relatively preserved phytohemagglutinin as opposed to decreased pokeweed mitogen responses may be due to possibly preserved responses via CD2/phytohemagglutinin pathway. J Immunol. 1989 Mar 15;142(6):1874–1880. [PubMed] [Google Scholar]
- Holmberg K., Meyer R. D. Fungal infections in patients with AIDS and AIDS-related complex. Scand J Infect Dis. 1986;18(3):179–192. doi: 10.3109/00365548609032326. [DOI] [PubMed] [Google Scholar]
- Klein R. S., Harris C. A., Small C. B., Moll B., Lesser M., Friedland G. H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984 Aug 9;311(6):354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- Manca F., Habeshaw J. A., Dalgleish A. G. HIV envelope glycoprotein, antigen specific T-cell responses, and soluble CD4. Lancet. 1990 Apr 7;335(8693):811–815. doi: 10.1016/0140-6736(90)90935-x. [DOI] [PubMed] [Google Scholar]
- Pedersen M., Permin H., Norn S., Skov P. S. Determination of immunoglobulins responsible for histamine release induced by microbial antigens in AIDS patients. Allergy. 1989 Sep;44(7):460–466. doi: 10.1111/j.1398-9995.1989.tb04183.x. [DOI] [PubMed] [Google Scholar]
- Scaringi L., Marconi P., Boccanera M., Tissi L., Bistoni F., Cassone A. Cell wall components of Candida albicans as immunomodulators: induction of natural killer and macrophage-mediated peritoneal cell cytotoxicity in mice by mannoprotein and glucan fractions. J Gen Microbiol. 1988 May;134(5):1265–1274. doi: 10.1099/00221287-134-5-1265. [DOI] [PubMed] [Google Scholar]
- Schellekens P. T., Roos M. T., De Wolf F., Lange J. M., Miedema F. Low T-cell responsiveness to activation via CD3/TCR is a prognostic marker for acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus-1 (HIV-1)-infected men. J Clin Immunol. 1990 Mar;10(2):121–127. doi: 10.1007/BF00918194. [DOI] [PubMed] [Google Scholar]
- Spector N. H. Neuroimmunomodulation takes off. Immunol Today. 1990 Nov;11(11):381–383. doi: 10.1016/0167-5699(90)90147-2. [DOI] [PubMed] [Google Scholar]
- Tollemar J., Ringdén O., Holmberg K. Candida albicans: mannan and protein activation of cells from various human lymphoid organs. Scand J Immunol. 1989 Oct;30(4):473–480. doi: 10.1111/j.1365-3083.1989.tb02452.x. [DOI] [PubMed] [Google Scholar]
- Torosantucci A., Boccanera M., Casalinuovo I., Pellegrini G., Cassone A. Differences in the antigenic expression of immunomodulatory mannoprotein constituents on yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1990 Jul;136(7):1421–1428. doi: 10.1099/00221287-136-7-1421. [DOI] [PubMed] [Google Scholar]
- Torosantucci A., Palma C., Boccanera M., Ausiello C. M., Spagnoli G. C., Cassone A. Lymphoproliferative and cytotoxic responses of human peripheral blood mononuclear cells to mannoprotein constituents of Candida albicans. J Gen Microbiol. 1990 Nov;136(11):2155–2163. doi: 10.1099/00221287-136-11-2155. [DOI] [PubMed] [Google Scholar]
- Via C. S., Morse H. C., 3rd, Shearer G. M. Altered immunoregulation and autoimmune aspects of HIV infection: relevant murine models. Immunol Today. 1990 Jul;11(7):250–255. doi: 10.1016/0167-5699(90)90099-u. [DOI] [PubMed] [Google Scholar]