Abstract
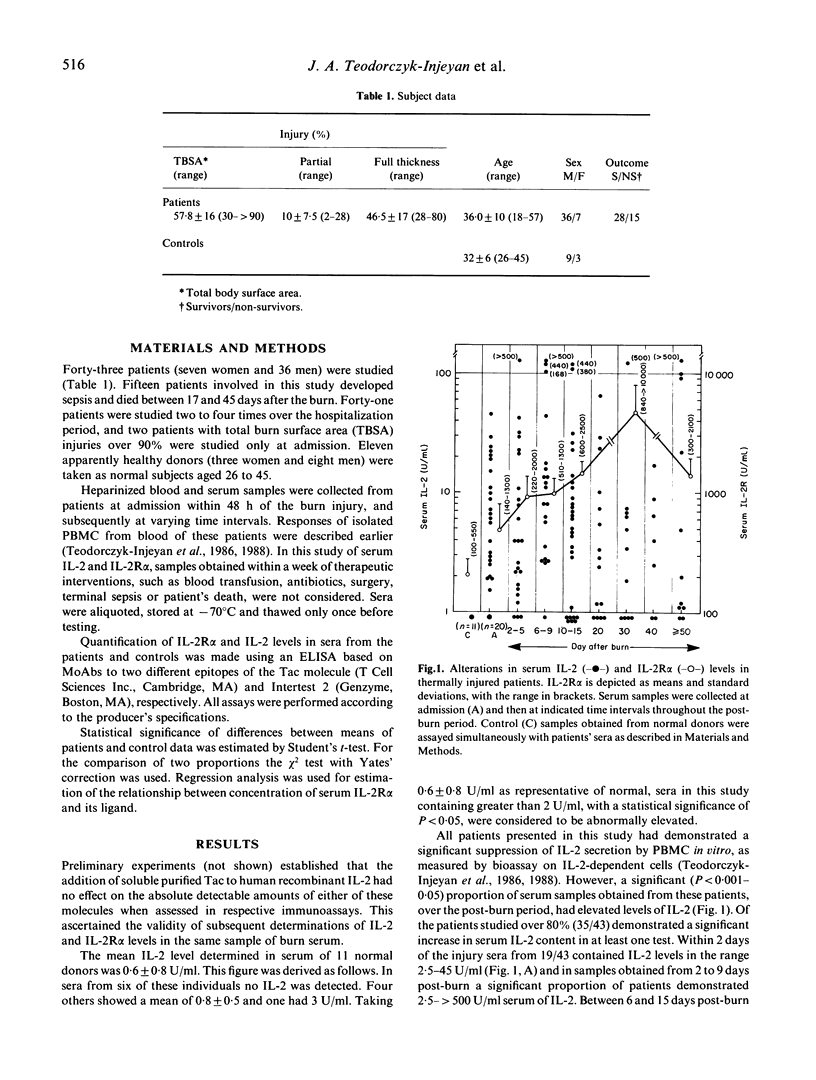
A general consensus that thermal injury affects T lymphocyte function adversely is supported particularly by the observation that burned patients' lymphocytes secrete reduced levels of biologically active IL-2 in vitro. In the same patients, however, high serum concentrations of the low-affinity IL-2 receptor (IL2R alpha), a product of an IL-2-activated gene, have been observed. In this study a significant proportion of patients also demonstrated over-physiological levels (from 2 to 500 U/ml) of serum IL-2 ascertained by immunoassay. Increases in serum IL-2 content correlated significantly (P less than 0.02) with those of serum IL-2R alpha during the first week post-burn. Later, serum IL-2R alpha levels continued to increase up to 30 days while IL-2 eventually declined. Thus, augmented secretion of IL-2R alpha appears related to the high serum IL-2 content. Therefore refractoriness to further immune stimulation may be due to early activation of the lymphoid system, rather than to an intrinsic incapacity of T lymphocytes for generating sequential responses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ades E. W., Bosse D., Orr S., Gillespie T. Immune responses in humans while receiving adoptive immunotherapy with recombinant interleukin-2 and lymphokine-activated killer cells: acute anergy to mitogens and recall antigens. Pathobiology. 1990;58(2):78–83. doi: 10.1159/000163566. [DOI] [PubMed] [Google Scholar]
- Antonacci A. C., Good R. A., Gupta S. T-cell subpopulations following thermal injury. Surg Gynecol Obstet. 1982 Jul;155(1):1–8. [PubMed] [Google Scholar]
- Calvano S. E., deRiesthal H. F., Marano M. A., Antonacci A. C. The decrease in peripheral blood CD4+ T cells following thermal injury in humans can be accounted for by a concomitant decrease in suppressor-inducer CD4+ T cells as assessed using anti-CD45R. Clin Immunol Immunopathol. 1988 May;47(2):164–173. doi: 10.1016/0090-1229(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Deitch E. A., Berg R. Bacterial translocation from the gut: a mechanism of infection. J Burn Care Rehabil. 1987 Nov-Dec;8(6):475–482. [PubMed] [Google Scholar]
- Deitch E. A., Xu D., Sitting K., Wohlman M., Bridges R. M., Landry K., McDonald J. C. Ficoll-Hypaque leukocyte preparations from burned patients contain activated nonlymphoid cell populations that take up thymidine. J Trauma. 1989 Mar;29(3):277–283. doi: 10.1097/00005373-198903000-00001. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Drogula C., Krönke M., Waldmann T. A., Greene W. C. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue J. H., Rosenberg S. A. The fate of interleukin-2 after in vivo administration. J Immunol. 1983 May;130(5):2203–2208. [PubMed] [Google Scholar]
- Granelli-Piperno A. In situ hybridization for interleukin 2 and interleukin 2 receptor mRNA in T cells activated in the presence or absence of cyclosporin A. J Exp Med. 1988 Nov 1;168(5):1649–1658. doi: 10.1084/jem.168.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel A. H., Travis W. D., Feinberg L., Pittaluga S., Striker L. M., Roberts W. C., Lotze M. T., Yang J. J., Rosenberg S. A. Pathologic findings associated with interleukin-2-based immunotherapy for cancer: a postmortem study of 19 patients. Hum Pathol. 1990 May;21(5):493–502. doi: 10.1016/0046-8177(90)90005-p. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Eberlein T. J., Spriggs D. R., Manogue K. R., Cerami A., Wilmore D. W. Interleukin-2 initiates metabolic responses associated with critical illness in humans. Ann Surg. 1988 Oct;208(4):493–503. doi: 10.1097/00000658-198810000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. L., Baker C. C. Changes in lymphocyte activity after thermal injury. The role of suppressor cells. J Clin Invest. 1979 Feb;63(2):202–210. doi: 10.1172/JCI109290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau G. J., Moldwin R. L., Lancki D. W., Kim D. K., Fitch F. W. Inhibition of IL 2-driven proliferation of murine T lymphocyte clones by supraoptimal levels of immobilized anti-T cell receptor monoclonal antibody. J Immunol. 1987 Jul 1;139(1):114–122. [PubMed] [Google Scholar]
- Rosenstein M., Ettinghausen S. E., Rosenberg S. A. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986 Sep 1;137(5):1735–1742. [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Sparkes B. G. Influence of burn-induced lipid-protein complex on IL2 secretion by PBMC in vitro. Burns. 1991 Apr;17(2):128–135. doi: 10.1016/0305-4179(91)90136-5. [DOI] [PubMed] [Google Scholar]
- Teodorczyk-Injeyan J. A., Sparkes B. G., Falk R. E., Peters W. J., Mills G. B. Interleukin-2 secretion and transmembrane signalling in burned patients. J Trauma. 1988 Feb;28(2):152–157. doi: 10.1097/00005373-198802000-00004. [DOI] [PubMed] [Google Scholar]
- Teodorczyk-Injeyan J. A., Sparkes B. G., Mills G. B., Falk R. E., Peters W. J. Impaired expression of interleukin-2 receptor (IL2R) in the immunosuppressed burned patient: reversal by exogenous IL2. J Trauma. 1987 Feb;27(2):180–187. doi: 10.1097/00005373-198702000-00015. [DOI] [PubMed] [Google Scholar]
- Teodorczyk-Injeyan J. A., Sparkes B. G., Mills G. B., Falk R. E., Peters W. J. Increase of serum interleukin 2 receptor level in thermally injured patients. Clin Immunol Immunopathol. 1989 May;51(2):205–215. doi: 10.1016/0090-1229(89)90020-2. [DOI] [PubMed] [Google Scholar]
- Teodorczyk-Injeyan J. A., Sparkes B. G., Mills G. B., Peters W. J., Falk R. E. Impairment of T cell activation in burn patients: a possible mechanism of thermal injury-induced immunosuppression. Clin Exp Immunol. 1986 Sep;65(3):570–581. [PMC free article] [PubMed] [Google Scholar]
- Teodorczyk-Injeyan J. A., Sparkes B. G., Peters W. J. Serum interleukin-2 receptor as a possible mediator of immunosuppression after burn injury. J Burn Care Rehabil. 1989 Mar-Apr;10(2):112–118. doi: 10.1097/00004630-198903000-00003. [DOI] [PubMed] [Google Scholar]
- Wolfe J. H., Wu A. V., O'Connor N. E., Saporoschetz I., Mannick J. A. Anergy, immunosuppressive serum, and impaired lymphocyte blastogenesis in burn patients. Arch Surg. 1982 Oct;117(10):1266–1271. doi: 10.1001/archsurg.1982.01380340002002. [DOI] [PubMed] [Google Scholar]
- Wood J. J., Rodrick M. L., O'Mahony J. B., Palder S. B., Saporoschetz I., D'Eon P., Mannick J. A. Inadequate interleukin 2 production. A fundamental immunological deficiency in patients with major burns. Ann Surg. 1984 Sep;200(3):311–320. doi: 10.1097/00000658-198409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood N. C., Symons J. A., Duff G. W. Serum interleukin-2-receptor in rheumatoid arthritis: a prognostic indicator of disease activity? J Autoimmun. 1988 Aug;1(4):353–361. doi: 10.1016/0896-8411(88)90005-4. [DOI] [PubMed] [Google Scholar]
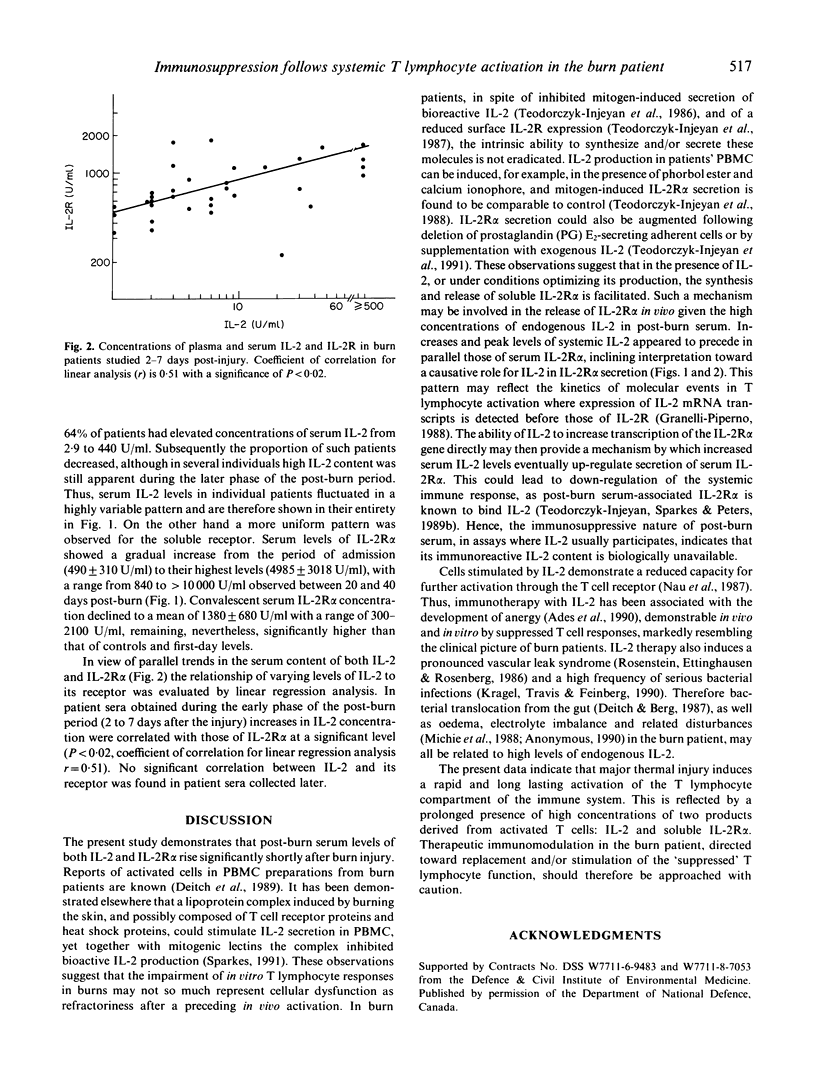
- Yasuda N., Lai P. K., Ip S. H., Kung P. C., Hinuma Y., Matsuoka M., Hattori T., Takatsuki K., Purtilo D. T. Soluble interleukin 2 receptors in sera of Japanese patients with adult T cell leukemia mark activity of disease. Blood. 1988 Apr;71(4):1021–1026. [PubMed] [Google Scholar]


