Abstract
A thin carbonate unit associated with a Sturtian-age (≈750–700 million years ago) glaciogenic diamictite of the Neoproterozoic Kingston Peak Formation, eastern California, contains microfossil evidence of a once-thriving prokaryotic and eukaryotic microbial community (preserved in chert and carbonate). Stratiform stromatolites, oncoids, and rare columnar stromatolites also occur. The microbial fossils, which include putative autotrophic and heterotrophic eukaryotes, are similar to those found in chert in the underlying preglacial units. They indicate that microbial life adapted to shallow-water carbonate environments did not suffer the significant extinction postulated for this phase of low-latitude glaciation and that trophic complexity survived through snowball Earth times.
Neoproterozoic low-latitude glaciation and its aftermath are commonly thought to have imposed an “environmental filter” on the course of evolution (1–3). The biologic crisis is postulated to have two distinct pulses: (i) a glacial “snowball Earth” phase characterized by severe low-latitude glaciation that was immediately followed by (ii) an intense greenhouse phase characterized by remarkable ocean chemistry that fostered the precipitation of unusual postglacial “cap carbonates” (1). These conditions were repeated as many as four or more times between ≈750 million years ago (Ma) and the Precambrian–Cambrian boundary, 543 Ma. The exact nature of snowball Earth conditions are the subject of considerable debate (e.g., ref. 4). Global snowball conditions might have included great extremes in diurnal and seasonal temperatures at all latitudes, mean annual temperatures well below freezing (possibly as low as −50°C) (5), and completely or generally ice-covered oceans (3). This intense freeze may have lasted as long as 10 million years (3). The postglacial conditions may have produced very warm surface temperatures (≈40°C) over a geologically short interval of time (see figure 7 in ref. 3) in concert with the global precipitation of the unusual cap carbonates and perturbations in the biogeochemical cycling in the ocean. Suggested effects on the biosphere range from the speculation that most eukaryotic lineages went extinct (3) to the notion of life surviving in refugia (3, 5, 6).
There is resistance to the hypothesized severity of snowball Earth conditions within the paleobiological community (e.g., ref. 5) and elsewhere (4). It is well known that several key clades of eukaryotic autotrophs (e.g., algae) and heterotrophs (e.g., testate amoebae) predate the first severe Neoproterozoic glaciation (known as the Sturtian glaciation, ≈750–700 Ma) and postdate the last glaciation (commonly termed the Marinoan or Varanger glaciation, ≈600 Ma) (7, 8). Most researchers agree that prokaryotic organisms (e.g., cyanobacteria and other bacteria) would have a high probability of surviving a snowball Earth event, because many living prokaryotes have adapted to, and thrive within, cold harsh environments (6, 9). However, with regard to the microfossil record, there are few microbiotas that immediately predate, are found within, and/or directly postdate snowball Earth glacial deposits (see ref. 10). We do know that a number of prokaryotic and eukaryotic clades survived (3), but did they experience severe or moderate extinction during the glacial events? Did surviving clades undergo radiation after the glacial events, as might be predicted with such an environmental crisis (e.g., ref. 7)? Can we observe evidence of the environmental filter (3) or “bottlenecks” (7) that have been hypothesized?
Here, we report the implications of diverse fossil microbiotas from the Death Valley region, California, found immediately preceding and within (or possibly capping) demonstrably glacial strata deposited during one of the Neoproterozoic snowball Earth episodes. Although diverse microbiotas are known from strata that immediately postdate snowball events (e.g., Tindir Group; see refs. 8 and 11) and some diverse microbiotas are known from strata that predate a snowball event (e.g., the Skillogalee Dolomite in South Australia; ref. 10), to our knowledge, these Californian fossil microbiotas provide the only direct evidence of diverse eukaryotic and prokaryotic microbial fossils that immediately predate and occur within Neoproterozoic glacial strata. These fossils provide the best details of the survival of prokaryotes and eukaryotes although a snowball Earth event. The Californian microfossils challenge the ideas that climatic perturbations catastrophically affected the marine biosphere and they suggest that a completely ice-covered ocean was unlikely, or that the resiliency of life has been underestimated.
Geologic Background
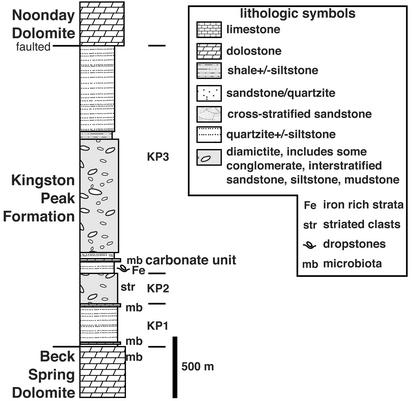
The Neoproterozoic Kingston Peak Formation (12–14) in which the key microbiotas occur overlies the Beck Spring Dolomite and underlies the Noonday Dolomite in the Kingston Range, eastern California, our primary study area. It is a heterolithic unit containing sandstone, siltstone, mudstone, conglomerate, breccia, and diamictite, as well as minor amounts of iron-formation, carbonates, and volcanics (Fig. 1). Locally, striated pebbles and dropstones indicate a glacial origin of some of the diamictites (12). Facies and thickness of units change rapidly across the basin, indicating deposition in an actively extending (15) and/or transpressional (16) tectonic environment probably related to the rifting of western North America in Neoproterozoic time. Olistoliths in excess of 1.5 km in length from underlying units occur in the Kingston Peak Formation; their origin is controversial (15–17). In the Panamint Range, the Kingston Peak Formation clearly contains two separate, glacially derived units (15). Each glacial unit is overlain by a cap carbonate that records a negative carbon isotopic anomaly (13, 18, 19), a pattern that is repeated in Neoproterozoic successions around the world (13, 18). The Kingston Peak Formation postdates the sills in the underlying Crystal Spring Formation dated at 1.087 billion years ago (14) and predates the Precambrian–Cambrian boundary found in the Wood Canyon Formation (20); thus, the age of the Kingston Peak Formation is rather poorly constrained. However, the lower glacial succession of interest here has been correlated to “Sturtian” age units (≈750–700 Ma) based on overall lithologic, chemostratigraphic, paleontologic, and chronostratigraphic comparison with similar successions elsewhere (13, 18). Attempts to determine the paleolatitude of the Kingston Peak Formation have been unsuccessful because of regional thermal overprints.§ However, paleomagnetic and chronologic constraints for the Chuar Group (21), Grand Canyon region, thought to be broadly coeval with and proximal to the Beck Spring Dolomite and lowermost preglacial Kingston Peak Formation,¶ indicate that the southwestern United States was between 5° and 10° latitude near 750 Ma. Thus, the Kingston Peak Formation glacial strata were probably deposited at low latitudes ≈750–700 Ma, although this assignment requires further investigation.
Figure 1.
Generalized stratigraphy of the Neoproterozoic Death Valley succession, Kingston Range, eastern California, at locality 1. Thicknesses are approximate (after ref. 42). The Kingston Peak Formation thins dramatically to the northwest at locality 2.
The Kingston Range Localities
In the Kingston Range, the Kingston Peak Formation has been divided into three informal packages: KP1 (mostly fine-grained, preglacial siliciclastics without diamictite), KP2 (mostly diamictite), and KP3 (iron-rich graded beds, olistolith-bearing diamictite, conglomerate, sandstone, siltstone, and breccia) (22) (Fig. 1). A particularly interesting fossiliferous, oncoid-bearing, dolomitic carbonate unit (hereafter called the “carbonate unit”) is found within KP3 (17, 23, 24) (Figs. 2, 3, and 4A). This 3-m-thick unit can be mapped over many kilometers in the Kingston Range, but it is not exposed elsewhere in the Death Valley region. It is commonly used as a regional marker bed (17) below the more chaotic olistolith-bearing unit. Regionally, it overlies, in stratigraphic order, a succession of (i) diamictite, (ii) iron-rich, dropstone-laden turbidites, and (iii) diamictite plus cross-stratified sandstone, siltstone, and laminated mudstone with dropstones (Fig. 1).
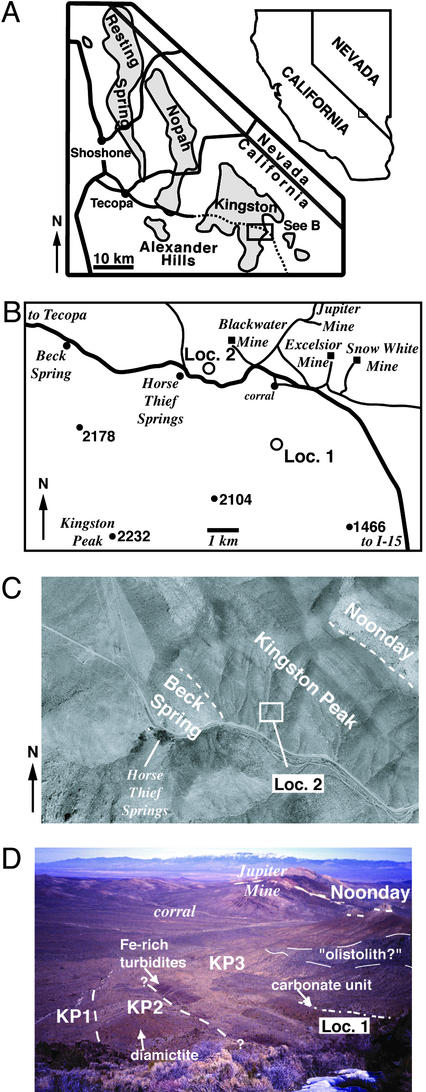
Figure 2.
Locality information for the study area, Kingston Range, eastern California. (A) Regional location map. Shaded area represents outcrop of Proterozoic–Lower Cambrian strata, after ref. 43. (B) Detailed location map for locality 1 (N35.75264°, W115.85711°) and locality 2 (N35.77425°, W115.88322°), based on U.S. Geological Survey 1:100,000-scale map of the Kingston Range. Numbered circles are prominent topographic peaks, with elevation in meters. (C) Aerial photograph of locality 2. (D) Field photograph of locality 1, view to the northeast toward the Jupiter Talc mine (white talc talus in distance) and Spring Mountains (high mountain range in background). The contact between KP2 and KP3 is approximate. Note large olistolith of Beck Spring Dolomite above the carbonate unit.
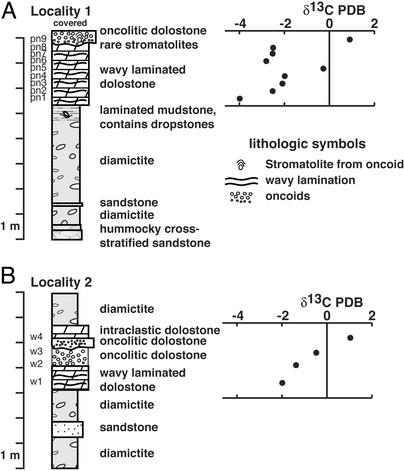
Figure 3.
Detailed lithostratigraphy and chemostratigraphy of the carbonate unit at locality 1 (A) and locality 2 (B). Lithologic symbols are the same as in Fig. 1. PDB, Peedee belemnite standard. Numbers to left of column correspond to isotopic analysis.
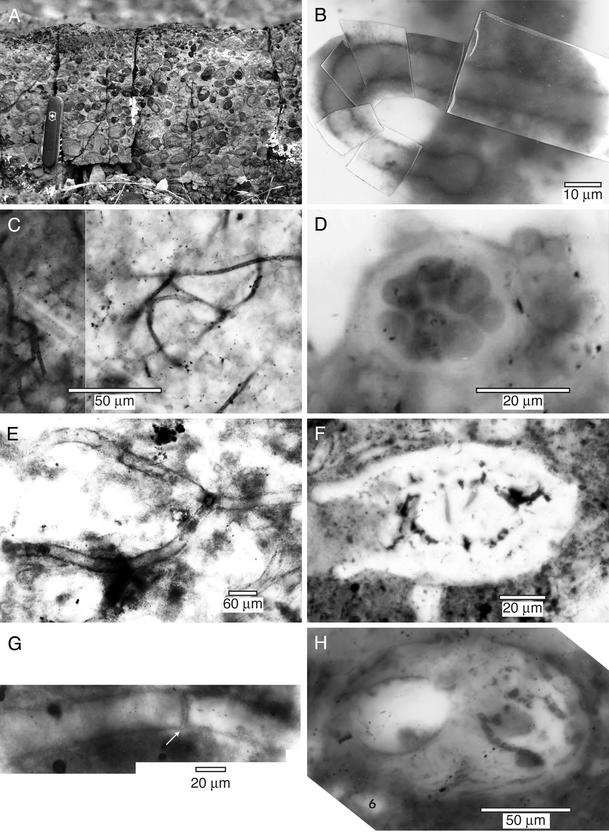
Figure 4.
Representative microfossils preserved in chert from the carbonate unit in the Neoproterozoic Kingston Peak Formation. (A) Field photo of upper portion (oncolite) of carbonate unit at locality 1, showing abundant oncoids. (B) Photomosaic of tubular structure with bulb at one end. (C) Filamentous microfossils (probable cyanobacteria). (D) Possible pluricellular cyanobacterium. (E) Large branching septate filament (Palaeosiphonella; probably an autotrophic eukaryote); width of filament is 30 μm. Close inspection demonstrates that the branching is characteristic of this organism and is not an artifact of superposition. (F) Melanocyrillium, probable testate amoebae (heterotrophic eukaryote); fossil is 117 μm across. (G) Unidentified tubular microfossils with double-walled partition (probably an autotrophic eukaryote). (H) Unidentified tubular structure with complex internal components.
We have studied the carbonate unit in detail at two localities (Figs. 2 and 3). At both localities, the contact between the unit and the underlying siliciclastic strata is sharp. At the base, the unit is finely laminated dolostone with wavy, stratiform stromatolitic laminae and contorted laminae. Silicified portions of the laminated dolostone preserve recognizable stromatolitic lamination (often with poorly preserved microfossils) as well as flat pebbles of microbial mat material. Chert has replaced carbonate in portions of the unit in the form of millimeter- to centimeter-thick beds, lenses, stringers, nodules, and polymorphic masses. At locality 1 (Fig. 3A), the carbonate unit is about 3 m thick. The underlying strata are composed of olive drab to black mudstone with dropstones that occur within 1 m of the contact. The carbonate unit here can be subdivided into two components: a lower wavy laminated (stratiform stromatolites) part and an upper oncolite. The contact between the wavy laminated portion and the oncolite is sharp and it displays erosional relief of ≈10 cm. The oncolite is composed of small, millimeter-scale oncoids at the base that grade into larger, centimeter-scale oncoids and rare centimeter-scale columnar stromatolites near the top. Individual oncoids are commonly preferentially silicified. The small columnar stromatolites, 5–10 cm tall, appear to have nucleated from stabilized oncoids. The contact between the carbonate unit and the overlying unit is obscured. At locality 2 (Fig. 3B), the carbonate unit is slightly thinner (about 2.5 m) and it rests with sharp, planar contact with the underlying diamictite. Here, the carbonate unit can be subdivided into four components. The lowest component is wavy laminated (stratiform stromatolites); however, the waviness is more disharmonic than found at locality 1. It is sharply overlain by the next component, an oncolite with centimeter-scale oncoids. This oncolite is sharply overlain by the third component, another oncolite bed (upper component) that contains smaller, millimeter-scale, oncoids. The fourth component is an intraclastic dolostone. The contact with an overlying diamictite appears conformable. The overlying diamictite appears virtually identical to the underlying diamictite in matrix and clast composition.
Because the paleoenvironmental indicators associated with the carbonate unit are subject to some latitude of interpretation in terms of depositional setting, we entertain two working hypotheses: (i) the carbonate unit represents a shallow marine environment, or (ii) the unit represents a nonmarine glacial environment akin to that found today in the Antarctic Dry Valley lakes (25). Because the carbonate unit lacks sedimentological (26), biogenic (27), or other (28) evidence to indicate a lacustrine origin, and a significant siliciclastic component, which is characteristic of the carbonate-containing sediments deposited within the Antarctic Dry Valley lakes (28), we favor a marine origin for these beds.
Chemostratigraphy
Isotope chemostratigraphy has become a widespread tool for the correlation of Neoproterozoic rocks (29–31). In particular, unusual carbonates (cap carbonates) that immediately overlie snowball Earth glacial units commonly record a strong negative δ13C anomaly (29, 32). Carbonate units that underlie the glacial units commonly record a positive to negative anomaly leading up to the glaciation (29), and interglacial carbonates record strongly positive δ13C values (e.g., ref. 29). For this part of the study, we collected samples for isotopic analysis from the two localities in portions that displayed the least silicification. Petrographic thin sections were prepared and examined in transmitted and polarized light as well as under cathodoluminescence to evaluate diagenetic alteration. Powders for analyses were microdrilled from portions of the least-altered, nonluminescent carbonate phases (usually microspar or micrite) and processed at the University of Maryland in the laboratory of A. J. Kaufman on a Micromass IsoPrime dual-inlet gas source stable isotope mass spectrometer with a peripheral MultiPrep system for on-line carbonate reactions. Uncertainties in this method of analysis are better than 0.05‰ for both carbon and oxygen isotopes. The data are plotted in Fig. 3 and included in Table 1. The oxygen isotopic composition of the samples averaged ≈−6‰ PDB, which is typical for little-altered Neoproterozoic carbonates (30). A δ13C isotopic trend, from ≈−4‰ PDB in the wavy laminated basal portion to ≈+1‰ at the top in association with oncoids and stromatolites, occurs in the carbonate unit at both sections [Fig. 3; note that this differs from the trends presented elsewhere (33), as we collected and plotted our samples in stratigraphic order]. It is noteworthy that the δ13C isotopic trend differs significantly from underlying units (see ref. 19), thus precluding the hypothesis that the carbonate bed represents an olistolith sourced from underlying units.
Table 1.
Carbon and oxygen isotopic data (relative to PDB standard) colected from the least-altered, petrographically screened samples at locality 1 and locality 2
| Sample | δ13C | δ18O |
|---|---|---|
| Locality 1 | ||
| pn1 | −4.0 | −2.1 |
| pn2 | −2.5 | −8.0 |
| pn3 | −2.1 | −5.8 |
| pn4 | −2.0 | −6.1 |
| pn5 | −0.3 | −5.7 |
| pn6 | −2.8 | −5.2 |
| pn7 | −2.5 | −11.2 |
| pn8 | −2.5 | −8.8 |
| pn9 | 1.1 | −2.2 |
| Locality 2 | ||
| w1 | −1.9 | −8.1 |
| w2 | −1.4 | −8.3 |
| w3 | −0.5 | −4.5 |
| w4 | 1.0 | −3.8 |
See Fig. 3 for location and stratigraphic position.
Death Valley Microbiotas
Microfossils are not uncommon in Beck Spring Dolomite and Kingston Peak Formation cherts (e.g., see refs. 24, 34, and 35). Chert for this study was collected in the Kingston Range from the uppermost Beck Spring Dolomite, carbonate beds in KP1, and the carbonate unit of KP3 (Fig. 1). Petrographic thin sections were prepared and examined with a Zeiss Photomicroscope II for microfossils by using transmitted and polarized light. Chert from the stratiform stromatolitic portions of all beds contains microbial remains; however, the preservation is usually poor. Silicified oncoids contain the best-preserved microfossils. Rare poorly preserved microfossils also occur in coexisting dolomite.
At least 12 distinctive microfossil morphotypes have been recognized within Beck Spring Dolomite and the Kingston Peak Formation; 11 occur in the carbonate unit (Fig. 4 and Table 2). In addition to filamentous (Fig. 4C) and coccoidal (Fig. 4D) morphologies interpreted as putative cyanobacteria (e.g., ref. 8), the biota contains several distinctive and significant noncyanobacterial microfossils. These include branched, septate, tubular microfossils (Fig. 4E) that are provisionally assigned to Palaeosiphonella (see ref. 34), tubular microfossils with double-walled partitions (Fig. 4G), a tubular structure with complex components (Fig. 4H), and vase-shaped microfossils (Fig. 4F). Vase-shaped microfossils are found in several Neoproterozoic formations and have been compared with testate amoebae (planktonic heterotrophs, ref. 7). Palaeosiphonella, which is probably an alga, is also reported from a preglacial unit in South Australia (10). The affinities of the double-partitioned form and the tubular structure with complex components are not yet known; however, they are probably eukaryotes on the basis of their large size and complex organization (double partition, complex internal features).
Table 2.
Biotic components
| Morphology | Scale, μm | CS | BS | KP
|
|
|---|---|---|---|---|---|
| KP1 | CU | ||||
| Coccoids | |||||
| Solitary | 4–56 | x | x | x | x |
| Loose cluster | 4–23 | x | x | x | x |
| Tight cluster, encompassing wall (cf. pleurocapsaleans) | 73 × 162 | x | x | ||
| Filaments | |||||
| Simple (Beckspringia sp.) | 1–6 | x | x | x | x |
| Sheath | 1–21 | x | x | x | x |
| Partitioned (Archaeonema sp.) | 2.5–4.5 | x | |||
| Probable eukaryotes | |||||
| Melanocyrillium sp. (vase-shaped) | >117 | x | x | x | |
| Tube with bulb | 10–20 | x | |||
| Tube with double partition | 20–40 | x | |||
| Spheroidal with complex features | 85–700 | x | x | ||
| Palaeosiphonella sp. | 20–40 | x | x | x | |
| Tenuocharta sp. (carbonate plate) | >100 | x | |||
CS, Crystal Spring Formation; BS, Beck Spring Dolomite; KP, Kingston Peak Formation (KP1; CU, carbonate unit). The scale of filamentous/tubular forms gives diameter of filament/tube. See Fig. 4 for photomicrographs of select microfossils. (See also refs, 23, 24, 34, and 35.)
The biotas from the uppermost Beck Spring Dolomite, the carbonate beds in KP1, and the carbonate unit are taxonomically and trophically diverse. The biota from the carbonate unit is possibly the most complex (it is also the best preserved) and represents both prokaryotic and eukaryotic primary producers as well as eukaryotic heterotrophs. Both benthic and planktonic microbes occur. This microbiota is quite similar in taxonomic and trophic structure to the preglacial microbiotas from KP1 and the uppermost part of the Beck Spring Dolomite (Table 2). It is similar to older microbiotas found in the lower part of the Beck Spring Dolomite and upper Crystal Spring Formation (24).
Discussion
The Carbonate Unit.
The similarity between the pre- and postcarbonate unit diamictites would argue for stratigraphic continuity and suggest that the carbonate unit was deposited during glacial times. Therefore, the microbiota from the carbonate unit would have been living during the glacial interval and it would constitute a snapshot of microbial life in a shallow marine environment. The isotopic composition of the carbonate unit would reflect aspects of Neoproterozoic glacial ocean chemistry during glacial times. Current snowball Earth models would preclude the presence of primary carbonates within the glacial deposits (1) and would suggest completely negative δ13C values for the glacial ocean because primary productivity was reduced/eliminated and mantle input of carbon at hydrothermal ridges (≈−6‰ PDB) dominated the carbon isotopic composition of the oceans. Hence, the Kingston Peak Formation carbonate unit indicates a more complex glacial ocean than predicted by these models because it records primary carbonates (in the form of coated grains, i.e., oncoids) and a negative to positive δ13C trend during the glaciation (see also ref. 33). Some workers have hypothesized that the negative δ13C anomalies recorded in the cap carbonates represent Rayleigh-type distillation during the transfer of CO2 from a CO2-supercharged atmosphere to the oceans and subsequent burial in cap carbonates in the immediately postglacial greenhouse (3). This model requires a high-CO2 atmosphere to drive ultimately the negative δ13C anomaly. However, we would not expect a high-CO2 atmosphere during the glaciation. Therefore, the negative δ13C anomaly recorded in the Kingston Peak Formation carbonate unit must have a different cause, and/or the previous models need revision.
The fact that the carbonate unit records a negative to positive δ13C excursion similar to the trend noted in cap carbonates (e.g., see refs. 3, 29, and 32), requires us to explore the possibility that the carbonate unit may be a cap carbonate rather than an intraglacial unit. In this case, the pre- and postcarbonate diamictites could represent different glacial events separated by an interval of carbonate deposition rather than continuous glaciogenic conditions. If this were the circumstance, the interglacial period would have been short, as evidenced by the nature of the lower and upper diamictites, which are virtually identical in all salient features (in particular clast composition). Because this is not the case, we prefer the synglacial hypothesis.
Significance of the Carbonate Unit Microbiota.
The microfossils from the Kingston Peak Formation carbonate unit are important in relation to snowball Earth hypotheses regardless of whether they are syn- or postglacial. The microbiota occurs above iron-formation and glacial units deposited at low latitude. The anoxia required for mobilization of iron (which later was precipitated as oxides to produce the iron-formation and iron-rich siliciclastic sediments) is postulated to result from complete or near complete ice cover (1–3). Therefore, the microbiota must have existed during or just after the peak of “snowball” glaciation rather than during some “presnowball” glacial buildup (2, 3) because the ice had reached low latitudes by this time. The fossil evidence does not support a major mass mortality, extinction of benthic or planktonic organisms (1, 2, 5, 37), or any other dramatic changes in the biosphere. The carbonate unit microbiota is similar to microbiotas found in the strata that immediately predate the glaciation (Fig. 1 and Table 2). The presence of photoautotrophic prokaryotes and photoautotrophic and heterotrophic eukaryotes in units that predate and co-occur with the glaciation indicates that morphotypes, trophic complexity, and taxonomic diversity survived through the glaciation without apparent disastrous effects. Finally, the similarity of the Kingston Peak Formation microbiota throughout the sections studied with the Beck Spring Dolomite microbiota diminishes the viability of the refugia hypothesis.
The refugia hypothesis contends that eukaryotes and prokaryotes survived in some unknown refugia where conditions remained more equitable and repopulated the postsnowball Earth from those refugia (3, 5). On the basis of the available paleontological data from the Death Valley region, we do not consider the refugia hypothesis tenable. The fact that the microbial components of the pre- and the syn- or postglacial microbiotas from Death Valley are nearly identical argues against adaptation in refugia. The likelihood of the same taxa and community structure that existed before the onset of glaciation adapting to, and surviving in, refugia unchanged, and then repopulating the marine environment is remote.
The snowball Earth hypothesis predicts a “freeze-fry, double-whammy” (38) to have affected the course of evolution in a bottleneck and flush style (a complete global freeze followed by an intensely hot and inhospitable aftermath). The Kingston Peak Formation microbiotas contradict these hypothesized extremes. Taxonomic and trophic complexity does not appear to have changed significantly during the glaciation. A similar absence of change is noted for the Marinoan glaciation (≈600 Ma) in Australia.‖ Thus, we conclude that a “hard snowball” (completely ice-covered oceans) followed by an inhospitably warm period is not consistent with the fossil evidence. Rather, we conclude that a “soft snowball” (with ice-free tropics) followed by a warm (but not inhospitable) period is more consistent with the evidence found in the Kingston Peak Formation. Whereas the paleomagnetic evidence for low-latitude glaciation in Neoproterozoic times is quite robust (39), the extent of sea-ice cover (4), and thus the potential to affect drastically the course of evolution, is less clear. Simpler global climate models have predicted completely ice-covered oceans (40), but more complex models have failed to predict complete freezing of tropical oceans (4, 41).
Conclusion
Microbiotas from the Kingston Peak Formation provide a unique window into Neoproterozoic biotic diversity and trophic structure surrounding one major episode of snowball Earth. No significant changes are noted between older, presnowball biotas and the synglacial snowball biotas. The fact that heterotrophic and autotrophic eukaryotes appear to have survived unscathed through this interval leads one to question the severity of glacial environments in the tropical marine realm during these glacial times, the amount of ice-covered oceans, and the inhospitable nature of the snowball aftermath. While we must accept the credibility of low-latitude glaciation in Neoproterozoic time, and accept that these conditions represented a stressful environment on many parts of the globe, the extent of ice-covered oceans, and thus the ability to affect severely the course of evolution, is less clear.
Acknowledgments
We thank Dave Bottjer, Al Fischer, Grant Young, James W. Hagadorn, and four anonymous reviewers for examining versions of this manuscript, and Dr. A. J. Kaufman for isotopic expertise. We thank Dr. J. C. Crowell for guidance and encouragement over the years.
Abbreviations
- Ma
million years ago
- PDB
Peedee belemnite
Footnotes
Gillett, S. L., Kirschvink, J. L., van Alstine, D. R., Lewis, R. E. & Shoemaker, E. M. (1985) American Geophysical Union Abstracts 66, 876.
Dehler, C., Prave, A., Crossy, L., Karlstrom, K., Atudorei, V. & Porter, S., GSA Rocky Mountain and South-Central Joint Annual Meeting, May 7–9, 2001, Cedar City, UT, Vol. 33, no. 5, p. 20 (abstr.).
Grey, K. (2001) Geological Society of Australia Abstracts (Perth) 65, 45–47.
References
- 1.Hoffman P F, Kaufman A J, Halverson G P, Schrag D P. Science. 1998;281:1342–1346. doi: 10.1126/science.281.5381.1342. [DOI] [PubMed] [Google Scholar]
- 2.Kirschvink J L. In: The Proterozoic Biosphere: A Multidisciplinary Study. Schopf J W, Klein C, editors. Cambridge, U.K.: Cambridge Univ. Press; 1992. pp. 51–52. [Google Scholar]
- 3.Hoffman P F, Schrag D P. Terra Nova. 2002;14:129–155. [Google Scholar]
- 4.Hyde W T, Crowley T J, Baum S K, Peltier W R. Nature. 2000;405:425–429. doi: 10.1038/35013005. [DOI] [PubMed] [Google Scholar]
- 5.Runnegar B. Nature. 2000;405:403–404. doi: 10.1038/35013168. [DOI] [PubMed] [Google Scholar]
- 6.Vincent W F, Gibson J A E, Pienitz R, Villeneuve V, Broady P A, Hamilton P B, Howard-Williams C. Naturwissenschaften. 2000;87:137–141. doi: 10.1007/s001140050692. [DOI] [PubMed] [Google Scholar]
- 7.Porter S M, Knoll A H. Paleobiology. 2000;26:360–385. [Google Scholar]
- 8.Allison C W, Awramik S M. Precambrian Res. 1989;43:253–294. [Google Scholar]
- 9.Thomas D N, Dieckmann G S. Science. 2002;295:641–644. doi: 10.1126/science.1063391. [DOI] [PubMed] [Google Scholar]
- 10.Schopf J W. In: The Proterozoic Biosphere: A Multidisciplinary Study. Schopf J W, Klein C, editors. Cambridge, U.K.: Cambridge Univ. Press; 1992. pp. 1055–1117. [Google Scholar]
- 11.Zhang Y, Yin L, Xiao S, Knoll A H. Permineralized Fossils from the Terminal Proterozoic Doushantuo Formation, South China. Bridgewater, MA: Paleontological Soc.; 1998. , Memoir 50. [Google Scholar]
- 12.Miller J M G. In: Geology of Selected Areas in the San Bernardino Mountains, Western Mojave Desert, and Southern Great Basin, California: Geological Society of America Codilleran Section Volume and Guidebook. Cooper J D, Troxel B W, Wright L A, editors. Shoshone, CA: Death Valley Publishing; 1982. pp. 155–164. [Google Scholar]
- 13.Prave A R. Geology. 1999;27:339–342. [Google Scholar]
- 14.Heaman L M, Grotzinger J P. Geology. 1992;20:637–640. [Google Scholar]
- 15.Miller J M G. Geol Soc Am Bull. 1985;96:1537–1553. [Google Scholar]
- 16.Walker J D, Klepacki D W, Burchfiel B C. Geology. 1986;14:15–18. [Google Scholar]
- 17.Troxel B W, McMackin M A, Calzia J P, Walker J D, Klepacki D W, Burchfiel B C. Geology. 1987;15:274–275. [Google Scholar]
- 18.Corsetti F A, Awramik S M, Pierce D L, Kaufman A J. Int Geol Rev. 2000;42:516–533. [Google Scholar]
- 19. Corsetti, F. A. & Kaufman, A. J. (2003) Geol. Soc. Am. Bull., in press.
- 20.Corsetti F A, Hagadorn J W. Geology. 2000;28:299–302. [Google Scholar]
- 21.Karlstrom K E, Bowring S A, Dehler C M, Knoll A H, Porter S M, Des Marais D J, Weil A B, Sharp Z D, Geissman J W, Elrick M B, et al. Geology. 2000;28:619–622. doi: 10.1130/0091-7613(2000)28<619:cgotgc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Troxel B W. California Division of Mines and Geology Special Report. 1967;92:33–41. [Google Scholar]
- 23.Awramik S M, Corsetti F A, Shapiro R. Bull San Bernardino County Mus. 2000;47:65–74. [Google Scholar]
- 24.Pierce D, Cloud P. Geomicrobiol J. 1979;1:295–309. [Google Scholar]
- 25.Lawrence M J F, Hendy C H. N Z J Geol Geophys. 1989;32:267–278. [Google Scholar]
- 26.Nedell S S, Andersen D W, Squyres S W, Love F G. Sedimentology. 1987;34:1093–1106. [Google Scholar]
- 27.Awramik S M. Facies. 1997;36:230. [Google Scholar]
- 28.Bohacs K M, Carroll A R, Neal J E, Mankiewicz P J. In: Lake Basins through Space and Time. Gierlowski-Kordesch E H, Kelts K R, editors. Tulsa, OK: Am. Assoc. Petroleum Geologists; 2000. , AAPG Studies in Geology no. 46, pp. 3–33. [Google Scholar]
- 29.Kaufman A J, Knoll A H, Narbonne G M. Proc Natl Acad Sci. 1997;94:6600–6605. doi: 10.1073/pnas.94.13.6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knoll A H. Precambrian Res. 2000;100:3–20. doi: 10.1016/s0301-9268(99)00067-4. [DOI] [PubMed] [Google Scholar]
- 31.Walter M R, Veevers J J, Calver C R, Gorjan P, Hill A C. Precambrian Res. 2000;100:371–433. [Google Scholar]
- 32.Kennedy M J, Runnegar B, Prave A R, Hoffmann K H, Arthur M A. Geology. 1998;26:1059–1063. [Google Scholar]
- 33.Kennedy M J, Christie-Blick N, Prave A R. Geology. 2001;29:1135–1138. [Google Scholar]
- 34.Licari G R. J Paleontol. 1978;52:767–792. [Google Scholar]
- 35.Horodyski R J, Mankiewicz C. Am J Sci. 1990;290A:149–169. [Google Scholar]
- 36.Ambrose G J, Flint R B, Webb A W. Precambrian and Paleozoic Geology of the Peale and Denison Ranges. Geological Survey of South Australia, Adelaide: Dept. of Mines and Energy; 1981. , Bull. 50. [Google Scholar]
- 37.Hoffman P F, Kaufman A J, Halverson G P. GSA Today. 1998;8:1–9. [Google Scholar]
- 38.Hoffman P, Schrag D. Sci Am. 2000;282(1):68–75. [Google Scholar]
- 39.Sohl L E, Christie-Blick N, Kent D V. Geol Soc Am Bull. 1999;111:1120–1139. [Google Scholar]
- 40.Budyko M I. Tellus. 1969;21:611–619. [Google Scholar]
- 41.Hyde W T, Crowley T J, Baum S K, Peltier W R. Nature. 2001;409:306. doi: 10.1038/35013005. [DOI] [PubMed] [Google Scholar]
- 42.Miller J M G, Troxel B W, Wright L A. In: South Coast Geological Society, 1988 Field Trip. Gregory J L, Baldwin E J, editors. Santa Ana, CA: South Coast Geol. Soc.; 1988. [Google Scholar]
- 43.Stewart J H. Upper Precambrian and Lower Cambrian Strata in the Southern Great Basin, California and Nevada. Reston, VA: U.S. Geological Survey; 1970. , USGS Professional Paper 620. [Google Scholar]