Abstract
At the synapse, presynaptic membranes specialized for vesicular traffic are linked to postsynaptic membranes specialized for signal transduction. The mechanisms that connect pre- and postsynaptic membranes into synaptic junctions are unknown. Neuroligins and β-neurexins are neuronal cell-surface proteins that bind to each other and form asymmetric intercellular junctions. To test whether the neuroligin/β-neurexin junction is related to synapses, we generated and characterized monoclonal antibodies to neuroligin 1. With these antibodies, we show that neuroligin 1 is synaptic. The neuronal localization, subcellular distribution, and developmental expression of neuroligin 1 are similar to those of the postsynaptic marker proteins PSD-95 and NMDA-R1 receptor. Quantitative immunogold electron microscopy demonstrated that neuroligin 1 is clustered in synaptic clefts and postsynaptic densities. Double immunofluorescence labeling revealed that neuroligin 1 colocalizes with glutamatergic but not γ-aminobutyric acid (GABA)ergic synapses. Thus neuroligin 1 is a synaptic cell-adhesion molecule that is enriched in postsynaptic densities where it may recruit receptors, channels, and signal-transduction molecules to synaptic sites of cell adhesion. In addition, the neuroligin/β-neurexin junction may be involved in the specification of excitatory synapses.
In the developing mammalian brain, cell recognition generates an ordered network of ≈1015 synapses, linking ≈1012 neurons. The extraordinary specificity of synaptic connections develops in four basic steps: axonal pathway selection, target area selection, synaptogenesis, and synapse stabilization and modulation (1). Molecular mechanisms of axonal pathway selection have been studied in great detail, resulting in the characterization of multiple classes of hierarchically organized cell-surface proteins (2, 3). In contrast, it is unclear how an arriving axon selects a particular neuron from a large number of possible postsynaptic targets, how pre- and postsynaptic proteins are recruited to the initial site of synaptic contacts, and how synaptic junctions are connected.
The steps of synaptic recognition and synapse formation are likely to involve interactions between cell-adhesion molecules. However, the molecules mediating and regulating these steps are unknown. One exception is the cadherin family of cell-surface molecules. N-cadherin and its intracellular binding partners α- and β-catenin are expressed in a parasynaptic manner flanking release sites and postsynaptic densities (4) and may be involved in the regulation of synaptic plasticity (5, 6). In addition, a novel family of cadherin-like neuronal receptor proteins (Cnr1–8) has been discovered (7). Cnr1 is found in postsynaptic densities of neocortical synapses and may participate in synaptogenesis and synapse maintenance. However, cadherins are homotypic cell-adhesion molecules and form symmetric junctions, whereas synapses are asymmetric. Although homotypic cell-surface proteins may well be involved in synaptic recognition and adhesion, the asymmetry of synapses is likely to be mediated by heterotypic transsynaptic signaling. In fact, heterotypic cell adhesion has to be postulated to explain certain aspects of synaptogenesis, such as the specific and differential recruitment of pre- and postsynaptic protein components to their respective subcellular compartments.
Neuroligins 1, 2, and 3 constitute a family of brain-specific membrane proteins whose structural and biochemical characteristics are indicative of a role in heterotypic cell adhesion (8, 9). Their large extracellular N-terminal domain is homologous to serine esterases. Neuroligins bind to certain splice variants of β-neurexins, which are members of a polymorphic, brain-specific family of cell-surface proteins (10, 11). The interaction between neuroligins and β-neurexins mediates cell adhesion (12). In addition, the intracellular C-terminal tail of neuroligins binds to PSD-95 (13), a PDZ-domain protein that is thought to be involved in the assembly and organization of signal-transduction complexes in postsynaptic densities (14). The first two PDZ domains of PSD-95 bind to the N-methyl-d-aspartate (NMDA)-receptor subunit NMDA-R2 and K+ channels whereas the third PDZ domain interacts with the C terminus of neuroligins.
The characteristics of neuroligins and neurexins gave rise to the hypothesis that the two proteins form intercellular junctions between neurons (15). The cytoplasmic interaction of neuroligins with PSD-95, which is itself a synaptic protein (16), suggested that this junction may correspond to the synapse. Thus, neuroligins may serve as synaptic cell-adhesion molecules that organize the postsynaptic assembly of protein complexes involved in signal transduction. However, it has been difficult to test this hypothesis. The precise localization of neuroligins or neurexins is still unknown because generating high-quality antibodies to neuroligins or neurexins has turned out to be unexpectedly difficult. Thus, it is unclear where their intercellular junction is located. We have now succeeded in raising mAbs to neuroligin 1 that could be used to address this critical question. Our results demonstrate that neuroligins are postsynaptic cell-adhesion molecules that may interact with β-neurexins to form synaptic junctions.
MATERIALS AND METHODS
Generation of mAbs and Biochemical Procedures.
A glutathione S-transferase (GST)-fusion protein containing the extracellular sequences of neuroligin 1 (residues 1–695) was used to raise mAbs in collaboration with BioGenes (Berlin). Antibodies were characterized by using brain homogenates, immunoprecipitations, and immunocytochemistry on various species, recombinant GST-fusion proteins, and transfected COS cells (8, 9). SDS/PAGE (17) and Western blotting (18) were performed according to published procedures. Antibodies to NMDA-R1, synaptotagmin 1 (Cl41.1), synaptophysin (Cl 7.2), PSD-95, and msec7 have been published (13, 19–21). Subcellular fractionations of rat brain were carried out as described (8). For the developmental studies, rats of the indicated ages were killed, the brain was homogenized in SDS/PAGE sample buffer, and brain proteins were analyzed by quantitative immunoblotting using a Fujifilm BAS-5000 to quantitate the immunoreactivity.
Morphology.
For light microscopic analyses, brains of Wistar rats (200–250 g) or C57/B6 mice (30–50 g) were fixed by transcardial perfusion with 0.5 liters of 4% paraformaldehyde in PBS for 30 min. Brains were removed, cryoprotected in 20% sucrose overnight at 4°C, and processed as published (22, 23). Cryostat sections were incubated with anti-neuroligin 1 mAb 4C12 (1:500) followed by a secondary biotin-conjugated anti-mouse antibody (DAKO). Immune complexes were visualized by using nickel-intensified 3,3′-diaminobenzidine (DAB) staining (24). Double-labeling immunofluorescence experiments were performed with mAb 4C12 (1:100), anti-GluR2/3 polyclonal antibody (6 μg/ml, Chemicon), and anti-GABAAβ mAb (clone bd17, 20 μg/ml, Boehringer Mannheim). Pre-embedding DAB-based immunoelectron microscopy and postembedding immunogold electron microscopy (25) were performed as published (22, 25). Quantitative analyses of immunogold labeling were performed on randomly chosen electron micrographs (×20,000 magnification; 140 photographs for neuroligin 1; 25 photographs for synaptophysin).
RESULTS
Generation of mAbs to Neuroligin 1.
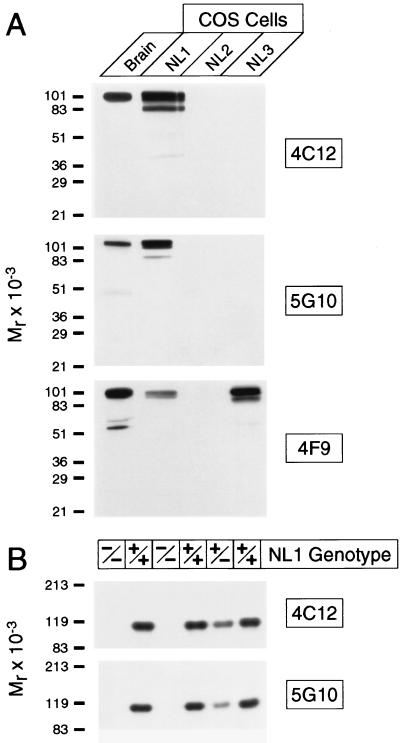
The polyclonal neuroligin antibodies that we had previously made were not useful for immunocytochemistry (8, 9). Therefore we used a recombinant fusion protein of the extracellular domain of neuroligin 1 to generate mAbs. We obtained three independent mAbs and tested their specificity by immunoblotting with recombinant neuroligins expressed in COS cells. Two of the three antibodies (4C12 and 5G10) reacted only with neuroligin 1, whereas the third (4F9) recognized neuroligins 1 and 3 but not neuroligin 2 (Fig. 1A). No other bands were detected. The isoform specificity of the antibodies was further probed by using neuroligin 1-deficient mice (Fig. 1B). These mice are viable and fertile, suggesting that neuroligin 1 is not an essential gene, possibly because of functional redundancy between neuroligins (N.B., R. E. Hammer, and T.C.S., unpublished data). When we analyzed the mutant mice with the neuroligin 1-specific antibodies 4C12 and 5G10, no immunoreactive band was detected in homozygous mutants, confirming that the antibodies exclusively recognize neuroligin 1.
Figure 1.
Specificity of mAbs to neuroligin. (A) Immunoblots of rat brain homogenates and COS cells expressing neuroligins 1, 2, and 3 (10 μg per lane) probed with mAbs 4C12, 5G10, and 4F9. (B) Immunoblots of mouse brain homogenates from homozygous (−/−) and heterozygous (+/−) neuroligin 1-mutant mice and wild-type control mice (+/+).
Light Microscopic Localization of Neuroligin 1.
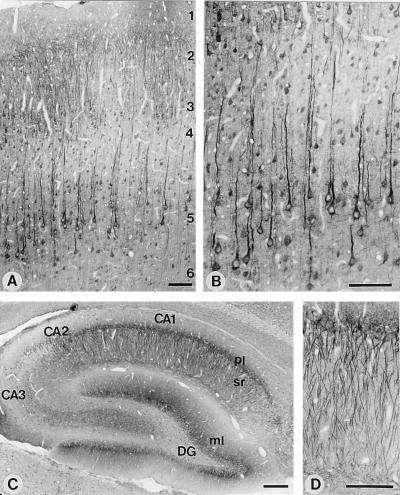
We next investigated whether the mAbs could be used for immunocytochemistry. Specific signals could be observed with mAbs 4C12 and 5G10 in rat or mouse brain (Fig. 2 and data not shown). Both antibodies gave identical staining patterns. However, 4C12 yielded a higher signal-to-noise ratio on sections and was employed for the detailed analyses described below.
Figure 2.
Light-microscopic localization of neuroligin 1 in rat brain. Shown are sections of the overall neocortex (A) or of layers 4 and 5 of the neocortex (B). Numbers indicate cortical layers. Low-magnification view of the entire hippocampal formation (C) or high-magnification view of the apical dendrites of pyramidal neurons from the CA1 region (D). DG, dentate gyrus; ml, molecular layer; pl, pyramidal cell layer; and sr, stratum radiatum. All sections were stained with antibody 4C12 and developed by using horseradish peroxidase-labeled secondary antibodies. [Bar = 100 μm (A, B, and D) or 250 μm (C).]
Previous in situ hybridization studies showed that neuroligins are expressed only in brain, where they are present in all neurons (10). In agreement with these results, we detected neuroligin 1 protein throughout the brain, where it was almost exclusively associated with neurons. Fig. 2 A and B show populations of pyramidal neurons in neocortical layers II–III and V–VI that display a uniform staining throughout individual somata as well as apical and, to a lesser extent, basal dendrites. In contrast, glial and endothelial cells were not significantly labeled. Very similar patterns of neuroligin 1 immunoreactivity were observed in hippocampal pyramidal cells (Fig. 2 C and D) and cerebellar Purkinje cells (data not shown). The immunoperoxidase staining pattern for neuroligin 1, with prominent labeling of neuronal cell bodies, closely resembles the pattern observed for PSD-95 (16) and the NMDA receptor subunit NMDA-R1 (26). To ensure the specificity of this staining pattern, we used the neuroligin 1-deficient mice. Specific labeling was abolished in mice lacking neuroligin 1, indicating that the observed staining pattern reflects the true distribution of neuroligin 1 (data not shown).
Colocalization and Coexpression of Neuroligin 1 with PSD-95 and NMDA-R1.
PSD-95 and NMDA-R1 are proteins that reside in the postsynaptic density, where they are thought to function in the transduction of the synaptic neurotransmitter signal (14). In addition to postsynaptic densities, both proteins are present in an intracellular vesicular pool, which explains the cell-body staining. The similarity between the staining patterns of neuroligin 1, PSD-95, and NMDA-R1 suggests that these three proteins may be colocalized in postsynaptic densities and intracellular vesicles. This was also suggested by subcellular fractionation experiments that revealed that neuroligin 1, PSD-95, and NMDA-R1 exhibit identical distributions (data not shown).
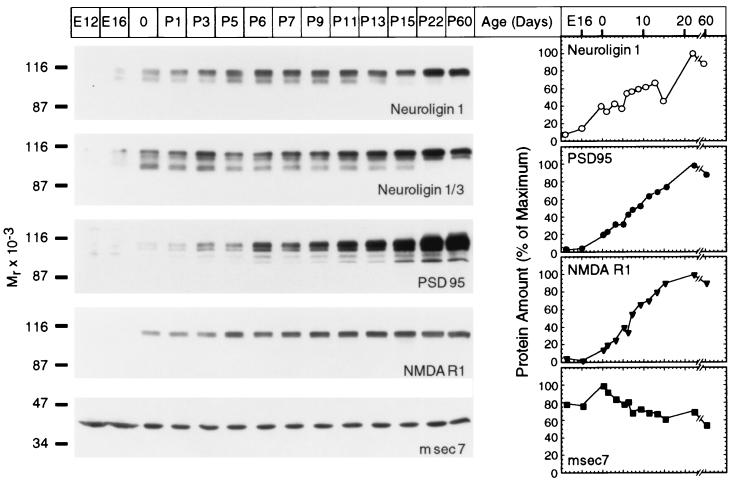
We next examined the pattern of developmental expression for neuroligin 1. Typically, levels of synaptic proteins increase with time after birth. This increase parallels the rate of synaptogenesis and, in the case of synaptic vesicle proteins, is largely caused by changes in posttranscriptional regulation (27). To explore whether neuroligin 1 behaves similarly to PSD-95 and NMDA-R1 in this respect, we measured the expression of neuroligin 1, PSD-95, and NMDA-R1 in rat brain as a function of age by using quantitative immunoblotting (Fig. 3). As a control we used msec7, a ubiquitous protein with a proposed role in Golgi transport that is not expected to change during development (20).
Figure 3.
Expression of neuroligin 1 in development. Brain homogenates from rats of the indicated age were analyzed by immunoblotting with antibodies to neuroligins, PSD-95, NMDA-receptor type 1 (NMDAR1), and msec7. Expression levels were quantified with iodinated secondary antibodies at the following ages: E12 and E16, embryonic days 12 and 16; 0, day of birth; P1–P60, postnatal days 1–60.
We found that the expression of neuroligin 1, PSD-95, and NMDA-R1 was coordinately up-regulated during development (Fig. 3). Neuroligin 1 expression was low in embryonic brains (embryonic days E12–E16) but increased dramatically after birth (postnatal days P0–P3). It reached a plateau during the period when most synapses are formed (P5–P8). This developmental-expression profile was paralleled by the NMDA-R1 receptor and PSD-95. Expression of msec7 was not altered (Fig. 3). Together these data suggest that neuroligin 1, PSD-95, and NMDA receptors are coexpressed in a coordinately regulated manner.
Localization of Neuroligin 1 by Using Quantitative Immunogold Electron Microscopy.
The light microscopy and biochemical results support the hypothesis that neuroligin 1 functions at the synapse and is colocalized with PSD-95 and NMDA-R1. To test this hypothesis, we analyzed the distribution of neuroligin 1 by postembedding immunogold electron microscopy (Fig. 4). To preserve the antigenicity of neuroligin 1, we performed these experiments with mildly fixed tissue in which many intracellular structures are difficult to recognize. Nevertheless, the characteristic shape of postsynaptic densities allowed easy identification of synapses.
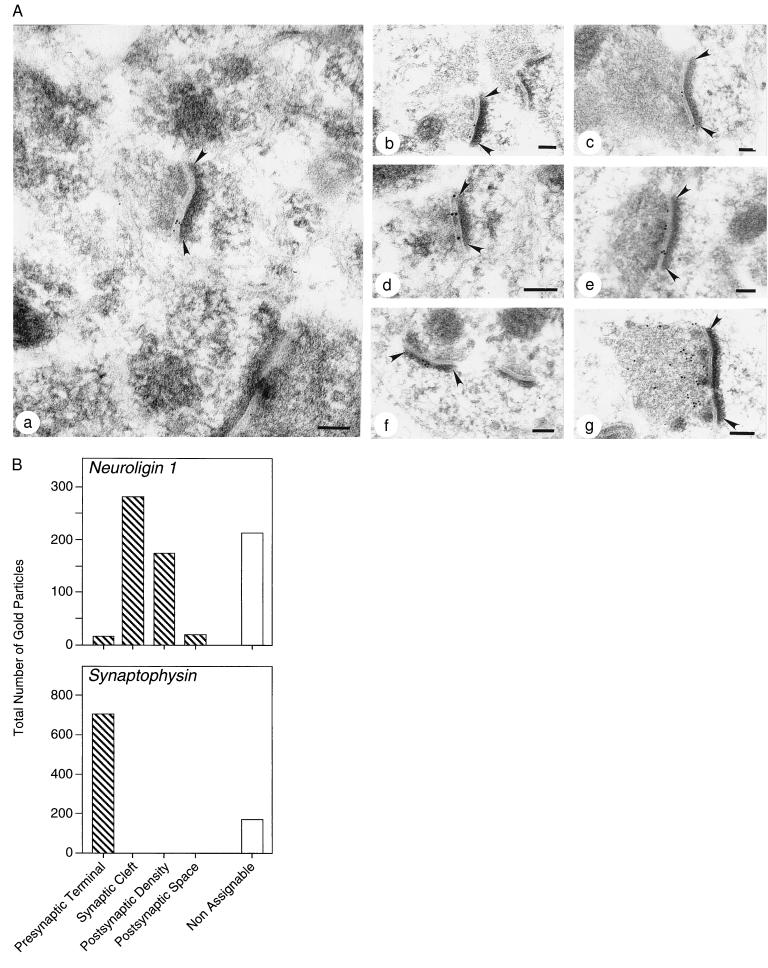
Figure 4.
Quantitative immunogold electron microscopy of neuroligin 1 and synaptophysin 1. (A) Representative ultrathin sections from rat neocortex stained with antibodies to neuroligin 1 (a–f) and synaptophysin (g). Neuroligin 1 immunoreactivity decorates synaptic clefts (a–e) and postsynaptic densities (f). Arrowheads indicate postsynaptic densities. (Bars = 0.1 μm.) (B) Quantitation of gold particles in randomly chosen sections labeled with antibodies to neuroligin 1 or synaptophysin. Total number of gold particles identified in the indicated subcellular compartments are given. Gold particles at locations that are not recognizable were designated nonassignable.
We observed a selective accumulation of immunogold particles in synaptic clefts and postsynaptic densities as a function of neuroligin staining (Fig. 4A). Only asymmetric synapses appeared to contain neuroligin 1. Typically, synapses were decorated with 2–6 gold particles in the synaptic cleft and/or adjacent postsynaptic densities. As our antibody was raised against the extracellular domain of neuroligin 1, the presence of gold particles in the synaptic cleft indicates that neuroligin 1 extends into the synaptic cleft. The clustering of gold particles in the postsynaptic density suggests that neuroligin 1 is (on average) closer to the postsynaptic side of the synapse. In contrast to neuroligin 1, antibodies to synaptophysin produced a completely different staining pattern (Fig. 4A). Synaptophysin is an abundant synaptic vesicle protein that is highly concentrated in presynaptic terminals (28). Immunogold labeling of synaptophysin yielded an exclusively presynaptic distribution of gold particles (Fig. 4A).
To ensure that neuroligin 1 is indeed synaptically localized, we analyzed the immunogold staining patterns quantitatively in a random set of electron micrographs (Fig. 4B). In neuroligin 1 stained sections, most gold particles (65%) were positioned in synapses. The remaining gold particles (35%) could not be attributed to identifiable structures and may correspond to intracellular vesicles or obliquely sectioned synapses. In synapses, 93% of gold particles resided in synaptic clefts or postsynaptic densities, demonstrating a highly specific enrichment of neuroligin 1 in these compartments. In contrast, immunogold labeling of synaptophysin was specific for presynaptic nerve terminals, which contained 80% of all gold particles (Fig. 4). The remaining 20% were not assignable to identifiable structures.
Double-Immunofluorescence Analysis of the Localizations of Neuroligin 1, Glutamate Receptors, and GABA Receptors.
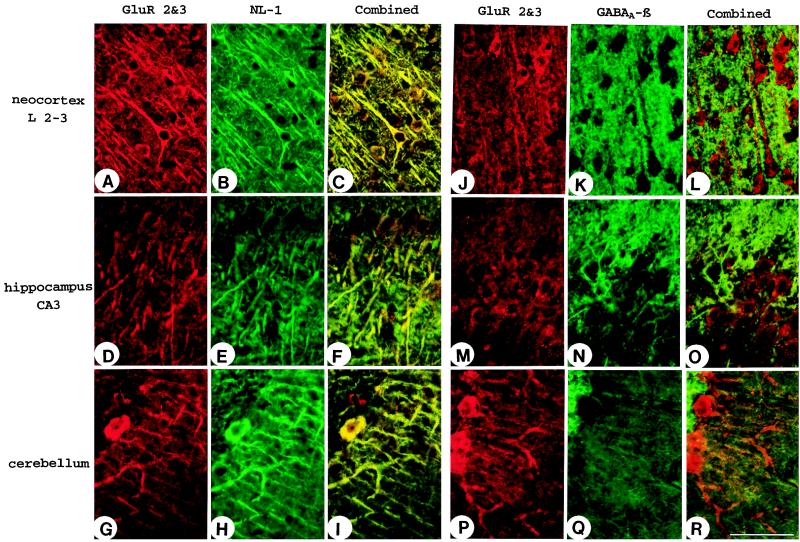
The immunogold electron microscopy suggested that neuroligin 1 is present primarily in asymmetric synapses, indicating that neuroligin 1 may be specific for excitatory synapses. To test this directly, we performed double-labeling experiments by using immunofluorescence with antibodies to markers for excitatory synapses (polyclonal antibodies to GluR2/3 glutamate receptors) and inhibitory synapses (mAbs to GABAAβ receptors). Neuroligin 1 was found to be colocalized with GluR2/3 in cortical and hippocampal pyramidal cells and in cerebellar Purkinje cells (Fig. 5). In contrast, double labeling of GABAAβ and GluR2/3 receptors resulted in the expected, mutually exclusive staining pattern with very little overlap (Fig. 5 J–R). Unfortunately, direct double labeling of brain sections for GABA receptors and neuroligin 1 was not possible with the antibodies to GABAergic markers available to us. Nevertheless, these data indicate that neuroligin 1 is preferentially targeted to excitatory synapses.
Figure 5.
Confocal micrographs of brain sections double labeled for neuroligin 1 and GluR2/3 glutamate receptors (A–I) or for GABAAβ receptors and glutamate receptors (J–R). Note the colocalization of GluR2/3 receptors (a marker for excitatory synapses) with neuroligin 1 in somata and dendrites of pyramidal neurons in neocortex and hippocampus and in Purkinje cells of the cerebellum (A–I). GABAAβ receptors, in contrast, are segregated (J–R). (Bar = 100 μm.)
DISCUSSION
Neuroligins constitute a family of neuronal cell-surface proteins. The structure of the neuroligins includes a large extracellular domain that is homologous to esterases but enzymatically inactive and a short cytoplasmic tail (8, 9). Neuroligins bind to β-neurexins, another class of neuronal cell-surface proteins (10, 11), in a manner that is regulated by the alternative splicing of β-neurexins. This binding reaction mediates cell adhesion between cells expressing neuroligins and β-neurexins, thereby creating an asymmetric intercellular junction. The neuronal expression of neuroligins and β-neurexins has led to the hypothesis that the junction they form may correspond to the synapse (8). In the current study, we provide evidence for this hypothesis. By using newly raised mAbs, we establish that neuroligin 1 is a synaptic cell-adhesion molecule. This conclusion is supported by the following results from the current and previous studies: (i) The neuronal localization of neuroligin 1 probed by immunoperoxidase labeling is indistinguishable from that of the postsynaptic marker proteins PSD-95 and the NMDA-R1 receptor. (ii) Neuroligin 1, PSD-95, and NMDA-R1 have similar subcellular distributions as analyzed biochemically. (iii) Neuroligin 1, PSD-95, and NMDA-R1 are coordinately expressed in development with large increases in expression during the period of postnatal synaptogenesis. (iv) PSD-95 physically interacts with both neuroligin 1 and NMDA receptors, thereby forming a cell-adhesion/glutamate receptor complex (13). (v) In quantitative immunogold electron microscopy, neuroligin 1 is clustered in postsynaptic densities and the synaptic cleft. (vi) Neuroligin 1 forms an asymmetric junction with β-neurexins belonging to a family of presumptive presynaptic latrotoxin receptors. (vii) In in situ hybridization experiments, neuroligin and neurexin mRNAs are only detectable in neurons (8, 11). (viii) Confocal microscopy showed that neuroligin 1 colocalizes with glutamatergic synapses but not GABAergic synapses, suggesting a specific targeting to excitatory synapses.
Heterophilic cell-adhesion molecules have not been identified in synapses before neuroligin 1, which is surprising considering the abundance and importance of synapses and their asymmetric nature. This contrasts with the multiple homophilic cell-adhesion molecules described in the vicinity of synapses, including cadherin-like receptors in vertebrates (7) and fasciclin II in Drosophila (29–31, and refs. therein). The subcellular localization of cadherin-like neuronal receptor 1 in postsynaptic densities and synaptic clefts is similar to that of neuroligin 1 (7). In Drosophila, transsynaptic cell adhesion at the neuromuscular junction may be mediated by fasciclin II (29–31). However, cadherins and fasciclin II are homotypic cell-adhesion molecules, whereas neuroligins are heterotypic cell adhesion molecules. This suggests that in vertebrates, cadherins and neuroligin have distinct synaptic functions, with neuroligins being related to the asymmetry of synapses.
Synapses are functionally complex and probably require multiple classes of cell-adhesion proteins for recognition of pre- and postsynaptic sides, specification of neurotransmitter type, structural cohesion, retrograde signaling, and many other properties. It is tempting to propose that the interactions of postsynaptic neuroligins with presynaptic β-neurexins constitute an intrinsic component of synaptic junctions that contributes to their structural stability. However, the current data indicate that neuroligin 1 does not simply form transsynaptic contacts between pre- and postsynaptic compartments but may be involved in the determination of synapse specificity, in distinguishing excitatory from inhibitory contact sites, and in recruiting protein components that are specific for excitatory synapses. The highly specific localization of neuroligin 1 is paralleled by the postsynaptic pool of its interaction partner PSD-95, which also is selectively targeted to excitatory synapses in cultured hippocampal cells (32), and by SynGAP, a novel cytosolic postsynaptic interactor of PSD95 (33, 34). These hypotheses, although supported by the available data, are far from proven. Precise localizations of neurexins as well as functional assays will be required to validate these ideas.
Acknowledgments
We thank S. Wenger, I. Leznicki, and A. Roth for excellent technical assistance, J. Ficner and L. Kolb for artwork, and R. Schubert for photographic work. We are grateful to Drs. M. S. Brown and J. L. Goldstein for innumerable discussions and suggestions. This work was supported by the Gerhard-Hess-Program of the German Research Foundation (Bonn, Germany) and by National Institute of Mental Health Grant RO1-MH50824.
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- NMDA
N-methyl-d-aspartate
References
- 1.Goodman, C. S. & Shatz, C. J. (1993) Cell72, Suppl., 77–98. [DOI] [PubMed]
- 2.Goodman C S. Annu Rev Neurosci. 1996;19:341–377. doi: 10.1146/annurev.ne.19.030196.002013. [DOI] [PubMed] [Google Scholar]
- 3.Chiba A, Keshishian H. Dev Biol. 1996;180:424–432. doi: 10.1006/dbio.1996.0316. [DOI] [PubMed] [Google Scholar]
- 4.Uchida N, Honjo Y, Johnson K R, Wheelock M J, Takeichi M. J Cell Biol. 1997;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue A, Sanes J R. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- 6.Tang L, Hung C P, Schuman E M. Neuron. 1998;20:1165–1175. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 7.Kohmura N, Senzaki K, Hamada S, Kai N, Yasuda R, Watanabe M, Ishii H, Yasuda M, Mishina M, Yagi T. Neuron. 1998;20:1137–1151. doi: 10.1016/s0896-6273(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 8.Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Moomaw C, Südhof T C. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 9.Ichtchenko K, Nguyen T, Südhof T C. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- 10.Ushkaryov Y A, Petrenko A G, Geppert M, Südhof T C. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich B, Ushkaryov Y A, Südhof T C. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen T, Südhof T C. J Biol Chem. 1997;272:26032–26039. doi: 10.1074/jbc.272.41.26032. [DOI] [PubMed] [Google Scholar]
- 13.Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl T W, Südhof T C. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 14.Kornau H, Seeburg P, Kennedy M. Curr Opin Neurobiol. 1997;7:386–373. doi: 10.1016/s0959-4388(97)80064-5. [DOI] [PubMed] [Google Scholar]
- 15.Missler M, Südhof T C. Trends Genet. 1998;14:20–26. doi: 10.1016/S0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- 16.Hunt C A, Schenker L J, Kennedy M B. J Neurosci. 1996;16:1380–1388. doi: 10.1523/JNEUROSCI.16-04-01380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brose N, Huntley G W, Stern-Bach Y, Sharma G, Morrison J H, Heinemann S F. J Biol Chem. 1994;269:16780–16784. [PubMed] [Google Scholar]
- 20.Telemenakis I, Benseler F, Stenius K, Südhof T C, Brose N. Eur J Cell Biol. 1997;74:143–149. [PubMed] [Google Scholar]
- 21.Butz S, Okamoto M, Südhof T C. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- 22.Song J-Y, van Marle J, van Norden C J F, Frederiks W M. Histochem Cell Biol. 1996;106:573–580. doi: 10.1007/BF02473272. [DOI] [PubMed] [Google Scholar]
- 23.Song J-Y, van Marle J, van Norden C J F, Frederiks W M. Eur J Cell Biol. 1996;71:277–285. [PubMed] [Google Scholar]
- 24.Rickmann M, Wolff J R. Neuroscience. 1995;67:977–991. doi: 10.1016/0306-4522(94)00615-c. [DOI] [PubMed] [Google Scholar]
- 25.Merighi A, Polak J M. In: Immunohistochemistry. Cuello A C, editor. New York: Wiley; 1993. pp. 229–264. [Google Scholar]
- 26.Siegel S J, Brose N, Janssen W G, Gasic G P, Jahn R, Heinemann S F, Morrison J H. Proc Natl Acad Sci USA. 1994;91:564–568. doi: 10.1073/pnas.91.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daly C, Ziff E B. J Neurosci. 1997;17:2365–2375. doi: 10.1523/JNEUROSCI.17-07-02365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navone F, Jahn R, Di Gioia G, Stukenbrok H, Greengard P, De Camilli P. J Cell Biol. 1986;103:2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis G W, Schuster C M, Goodman C S. Neuron. 1997;19:561–573. doi: 10.1016/s0896-6273(00)80372-4. [DOI] [PubMed] [Google Scholar]
- 30.Thomas U, Kim E, Kuhlendahl S, Ho Koh Y, Gundelfinger E D, Sheng M, Garner C C, Budnik V. Neuron. 1997;19:787–799. doi: 10.1016/s0896-6273(00)80961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zito K, Fetter R D, Goodman C S, Isacoff E Y. Neuron. 1997;19:1007–1016. doi: 10.1016/s0896-6273(00)80393-1. [DOI] [PubMed] [Google Scholar]
- 32.Rao A, Kim E, Sheng M, Craig A M. J Neurosci. 1998;18:1217–1229. doi: 10.1523/JNEUROSCI.18-04-01217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J H, Liao D, Lau L-F, Huganir R L. Neuron. 1998;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- 34.Chen H J, Rojas-Soto M, Oguni A, Kennedy M B. Neuron. 1998;20:895–904. doi: 10.1016/s0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]