Abstract
Human activated protein C (APC) is an antithrombotic, antiinflammatory serine protease that plays a central role in vascular homeostasis, and activated recombinant protein C, drotrecogin alfa (activated), has been shown to reduce mortality in patients with severe sepsis. Similar to other serine proteases, functional APC levels are regulated by the serine protease inhibitor family of proteins including α1-antitrypsin and protein C inhibitor. Using APC–substrate modeling, we designed and produced a number of derivatives with the goal of altering the proteolytic specificity of APC such that the variants exhibited resistance to inactivation by protein C inhibitor and α1-antitrypsin yet maintained their primary anticoagulant activity. Substitutions at Leu-194 were of particular interest, because they exhibited 4- to 6-fold reductions in the rate of inactivation in human plasma and substantially increased pharmacokinetic profiles compared with wild-type APC. This was achieved with minimal impairment of the anticoagulant/antithrombotic activity of APC. These data demonstrate the ability to selectively modulate substrate specificity and subsequently affect in vivo performance and suggest therapeutic opportunities for the use of protein C derivatives in disease states with elevated serine protease inhibitor levels.
Human protein C (PC) is a vitamin K-dependent plasma serine protease with a well characterized role in maintaining normal hemostatic balance (1–3). Human PC is produced and circulates in the blood as a zymogen that is activated by thrombin in complex with the integral membrane protein thrombomodulin. Activated PC (APC), along with its cofactor, protein S, proteolytically inactivates factors VIIIa and Va, thereby reducing the generation of factor Xa and thrombin, effectively attenuating the clotting cascade (2). The physiological importance of PC is illustrated by the variety and severity of thrombotic disorders associated with deficiencies in PC. For example, homozygous PC-deficient infants suffer from potentially fatal purpura fulminans (4, 5), and in families with heterozygous PC deficiency there is an association with venous thrombotic disease (6). Consistent with the biological importance of its role in hemostasis and inflammation (7), the activated recombinant form of PC, Xigris [drotrecogin alfa (activated)], was proven to reduce mortality in severe sepsis (8).
Similar to other serine proteases involved in blood coagu-lation, APC is inhibited by members of the serine protease inhibitor (SERPIN) family of proteins (9, 10). The major SERPINs pertinent to APC physiologically are PC inhibitor (PCI) and α1-antitrypsin (α1-AT) (10, 11). SERPINs regulate the level of APC via a suicide-inhibition mechanism. After the cleavage of a specific loop in the SERPIN (termed the “reactive-site loop”) by the target protease, a conformational change occurs in the SERPIN that “locks” the two molecules in an irreversible, covalent complex (12, 13). As a result, APC has a relatively short physiological and pharmacokinetic (PK) half-life of ≈20 min versus ≈10 h for the zymogen (14). Therefore, an engineered APC exhibiting increased resistance to SERPIN inactivation, while maintaining desirable anticoagulant, antifibrinolytic, and antiinflammatory activities, would have an extended PK profile and afford an effectively more potent compound that may reduce dosage levels for therapeutic applications. The advantages realized with such a variant could be even greater in disease states in which levels of SERPINs are elevated such as disseminated intravascular coagulation (DIC) and sepsis (15–17).
It is known for serine proteases that amino acid residues within the active-site architecture that interact directly with the substrate define the substrate specificity (18, 19). Because of these specific interactions, it is possible to alter substrate specificity for serine proteases in general (20, 21), and APC in particular (22, 23), by substitution of amino acids that form direct, energetically important contacts with the various substrate side chains. Therefore, we used a structure-based strategy for the design of SERPIN-resistant derivatives. The intent was to alter the specificity of APC such that variants retained the ability to proteolytically inactivate coagulation factors Va and VIIIa while being less likely to cleave reactive-site loops in α1-AT or PCI that lead to irreversible inactivation. Although a number of variants exhibited some improvements, substitutions at Leu-194 (numbered beginning with the mature N terminus) were of particular interest, because they had substantially improved resistance to SERPIN inactivation that in turn resulted in a significant increase in the PK exposure. Importantly, functional characterization of the Leu-194 variants revealed that they exhibited relatively modest effects on proteolytic and anticoagulant functionality, demonstrating a shift in the proteolytic specificity.
Coupling the knowledge of key biological interactions to a structure-based drug design provides opportunities to tailor protein-based therapeutic agents for superior performance and therefore expanded clinical utility. We have shown for PC that engineered SERPIN resistance potentially can provide both. Notably, these results demonstrate that substantial changes in the biological profile can be attained with relatively minor changes in the protein structure, i.e., important physical interactions that determine specificity at the level of single residue–residue contacts within protein–protein interactions ultimately can have a large role on the biological activity and therefore can be rationally engineered with significant implications to the promise of new protein drug therapies arising from genomics efforts.
Materials and Methods
Materials.
Restriction endonucleases, cell-culture media, reagents, transfection reagents, and cloning enzymes were obtained from GIBCO/Life Technologies (Carlsbad, CA). Vitamin K1, α1-AT, poly-D-lysine, and methotrexate were purchased from Sigma. Activated partial thromboplastin time (aPTT) (kaolin) reagent and citrated, pooled human plasma (S.A.R.P.) were obtained from Helena Laboratories. Heparin sodium injection was from Elkins-Sinn (Philadelphia). Chromogenic substrate l-pyroglutamyl-l-prolyl-l-arginine-p-nitroanilide (S2366) specific for PC was from Chromogenix (Milan). Plasma-derived PCI was from Enzyme Research Laboratories (South Bend, IN). Factor Va was from Haematologic Technologies (Burlington, VT). Phospholipids were purchased from Avanti Polar Lipids. All other reagents were of the highest quality available.
Structural Modeling.
Structural models of APC–substrate complexes were created by using both the sequence corresponding to Arg-506 cleavage site in factor Va and the reactive-site loop for α1-AT (Fig. 1). The positions for the substrate and SERPIN sequence were determined by structural superposition of the D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone-inhibited APC crystal structure (PDB ID code 1AUT; ref. 24) with hirulog-3-inhibited thrombin (PDB ID code 1ABI; ref. 25). By using the positions of the hirulog sequence, amino acid side chains were substituted for the appropriate sequences, and the final structures were energy-minimized by using force-field calculations with the CHARMM (chemistry at Harvard molecular mechanics) program (26) (Molecular Simulations, Waltham, MA, using the incorporated parameter file for CHARMM 22) on a Silicon Graphics (Mountain View, CA) workstation.
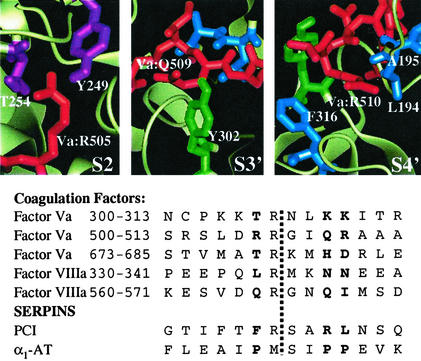
Figure 1.
APC specificity subsite identification. (Lower) The APC cleavage sites in both coagulation factors and SERPINs are aligned around the known cleavage site (indicated by the dashed vertical line), and residues corresponding to the S2, S3′, and S4′ subsites (39) are in bold. (Upper) The local structures of the three selected specificity subsites (39), S2, S3′, and S4′, are shown using the model of the APC:Va Arg-506 cleavage sequence; the substrate side chains are shown in red, and residues explored by mutagenesis are colored in magenta (S2), green (S3′), and blue (S4′), with the remainder of the APC protease domain shown in faded white. The figure was generated by using QUANTA 2000 (Molecular Simulations).
Variant Production and Characterization.
By using the 1,425-bp full-length human PC wild-type (wt) cDNA (27) as a template, mutations were made by PCR. Variant cDNAs were confirmed by sequencing before cloning into a eukaryotic expression vector pIG3, a derivative of pGT-d (28, 29). pIG3 was engineered by insertion of an internal ribosome entry site (IRES) (30) and GFP (31), resulting in expression of a bicistronic mRNA (5′-cDNA-IRES-GFP-3′). Coupling the cDNA and reporter on a single mRNA, translated as separate proteins, allowed us to screen for the highest-producing clones on the basis of GFP fluorescence intensity.
Syrian hamster tumor AV12-RGT18 cells (32) were cultured in growth medium (DMEM supplemented with 10% FBS/50 μg/ml gentamycin/200 μg/ml geneticin/10 μg/ml vitamin K at 37°C in 5% CO2). Linearized expression vectors were transfected into AV12-RGT18, and ≈48 h posttransfection the cells were split 1:10 into selection medium (growth medium supplemented with 0.25 μM methotrexate) and fed twice a week until the resistant colonies were pooled and expanded for screening. These pools were subjected to fluorescence-activated cell sorting based on GFP fluorescence intensity (31) with the most intense 5% of fluorescent cells being retained and expanded into roller-bottle culture for protein production and purification as described (33).
Activation of the zymogens was accomplished as described (34). Proteins were quantified rigorously and either used directly or frozen in aliquots at −80°C. SDS/PAGE, (35) Western blot, and HPLC analyses were used to confirm purity and complete activation. Amidolytic activities were determined by using the tripeptide substrate S2366 and a Molecular Devices ThermoMax kinetic microtiter plate reader. Kinetic constants for S2366 hydrolysis were determined as described (34). Anticoagulant activity was determined by using an aPTT clotting assay. Clotting reactions were monitored by using the ThermoMax kinetic microtiter plate reader, measuring the time to Vmax in the change in turbidity as the clot formed over time. Proteolytic inactivation of phospholipid-bound factor Va by APC variants was performed as described (36).
Inhibition of APC Derivatives in Plasma.
Inactivation of APC derivatives and plasma half-lives were determined by incubating 20 nM APC in citrated, pooled human plasma [90% (vol/vol)] at 37°C. Aliquots were removed at selected times, and residual APC amidolytic activity was measured by using S2366. The half-life (t1/2) in plasma was determined by nonlinear regression analysis of the decay curves generated by using SIGMAPLOT 2000 and the equation t1/2 = 0.69/k1(app) where k1(app) = the apparent first-order rate constant for inactivation. In addition, 10 units/ml heparin was added to similar reactions to assess the impact of PCI on the inactivation of APC in pooled human plasma. This heparin concentration was determined empirically to provide a maximal increase in the rate of inactivation. Experiments were also performed by using citrated plasmas from rabbits and cynomolgus monkeys (pooled from three animals of each species) before PK studies.
Inhibition by Purified SERPINs.
Inactivation rates by α1-AT were determined under pseudo-first-order kinetic conditions by incubation of 20 nM of each APC with a large excess of varying concentrations of α1-AT in activation buffer (AB)/BSA (20 mM Tris, pH 7.4, containing 150 mM NaCl and 1 mg/ml BSA). Aliquots were removed at selected times, residual APC amidolytic activity was measured by using S2366, and t1/2 was calculated as described above. Apparent first-order rate constants, k1, were calculated from the slopes of the plots of APC over time in the presence of a molar excess of the inhibitor as described (37), and second-order association rate constants, k2, were determined from the slope of the straight line obtained from a plot of k1 versus [α1-AT]. Rates of inhibition by PCI, in the absence or presence of heparin (10 units/ml), were measured similarly; 20 nM of each APC was incubated with 200 nM PCI at room temperature in AB/BSA. At selected times, aliquots were removed, and residual APC amidolytic activity was measured.
PK Studies.
PK experiments were performed in both normal rabbits and cynomolgus monkeys. New Zealand White rabbits (Myrtle's Rabbitry, Thompson Station, TN) were anesthetized briefly with isoflurane (5% for induction and 3% for maintenance), and catheters were implanted in the marginal ear vein (Becton Dickinson, Angiocath, 24 gauge) for drug administration and the central ear artery (Becton Dickinson, Insyte, 22 gauge) for blood collection. Rabbits were allowed to recover for 1–2 h before drug treatment. For cynomolgus monkey studies, drug was administered intravenously via the saphenous vein (Covance Laboratories, Madison, WI), and blood was collected by venipuncture via the saphenous vein. Solutions of wt and variant APCs (300 μg/ml) in buffer (20 mM Tris, pH 7.4, containing 150 mM NaCl) were administered in a bolus dose of 100 μg/kg intravenously in the rabbit (n = 3) and monkey (n = 2). Blood was collected into syringes or tubes containing 0.05 ml of 3.8% citrate and 0.5 M benzamidine to a final concentration of 1 part citrate/benzamidine:9 parts blood. Samples were collected at selected times posttreatment, spun as soon as convenient after collection to isolate plasma, and frozen. The plasma levels of APCs were determined by using an enzyme-capture assay as described (38), compared with standards made by dilution of the same protein into the appropriate plasma type. The Institutional Animal Care and Use Committees at Lilly Research Laboratories and Covance Laboratories approved the study protocols.
Results
Variant Design.
An examination of the cleavage sequences in factors Va and VIIIa and the recognition sequences in α1-AT and PCI revealed differences that were taken advantage of in terms of shifting the proteolytic specificity of APC (Fig. 1). For example, the two SERPIN sequences contain hydrophobic side chains that occupy the S2 and S4′ specificity pockets (i.e., two residues N-terminal to and four residues C-terminal to the cleavage site; ref. 39), whereas the Va and VIIIa cleavage sites contain predominantly polar or charged side chains. To identify the residues in APC that form key contacts at these sites, two APC–substrate structural models were created. Starting with the published x-ray crystal structure of APC (24), two different substrate sequences were modeled into the active site by using the hirulog-3–thrombin complex crystal structure (25) as a template for determining the approximate positions of the substrate side chains on the C-terminal side of the cleavage site, i.e., the S′ specificity sites. The sequences chosen corresponded to the Arg-506 cleavage site in factor Va and the reactive-site loop in α1-AT, comprising residues four positions before the cleavage site through four positions after the cleavage site. Although minor differences in side-chain positioning were observed between the two models, the APC residues forming each of the specificity pockets were consistent. Based on these models, a number of positions were selected for mutagenesis, focusing on the S2 and S4′ specificity pockets (Fig. 1). For example, to disfavor recognition of hydrophobic side chains at these subsites, mutations were made such that the resulting side chain was more polar (e.g., Thr → Ser or Leu → Ser).
Inhibition in Human Plasma.
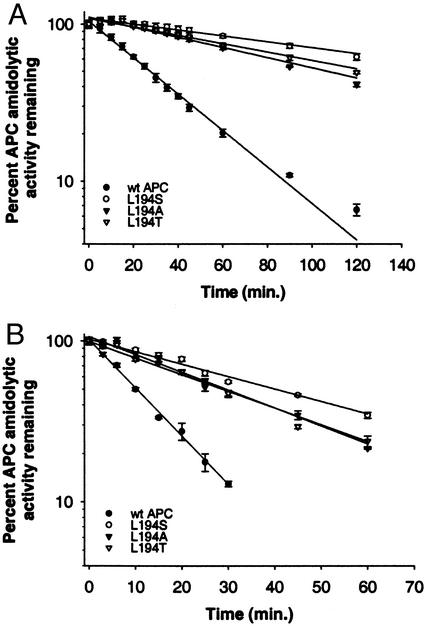
As a surrogate measure of resistance to inhibition by α1-AT and/or PCI, the inactivation half-lives of wt and derivative APC molecules in human plasma were investigated and are summarized in Table 1. The half-life of 26 min measured for wt APC was similar to that reported previously (14). Although all but the F316N variant exhibited slower inactivation in human plasma, the Leu-194 variants were remarkable in their ability to resist inactivation (Fig. 2A); the single substitutions at Leu-194 resulted in 4- to 6-fold increases in plasma half-life compared with wt APC, with L194S being the most resistant to inactivation. Not surprisingly, combining mutations bestowed effects that were even greater as demonstrated by the L194A/T254S or L194S/T254S double mutants (Table 1).
Table 1.
Half-lives in pooled human plasma plus or minus heparin and aPTT
| Variant | t1/2, min (no heparin) | t1/2, min (10 units/ml heparin) | aPTT time, sec |
|---|---|---|---|
| wtAPC | 26.0 ± 0.8 | 10.1 ± 0.9 | 83 ± 5 |
| L194S | 160.4 ± 14.8 | 38.9 ± 2.6 | 71 ± 7 |
| L194A | 93.0 ± 7.6 | 27.1 ± 2.2 | 74 ± 6 |
| L194T | 113.1 ± 7.9 | 29.0 ± 2.5 | 78 ± 11 |
| A195G | 48.7 ± 1.7 | ND* | 86 ± 8† |
| T254S | 49.5 ± 5.2 | ND | 82 ± 5† |
| F316N | 28.7 ± 0.9 | ND | 73 ± 4† |
| Y302Q | 41.6 ± 1.4 | ND | 60 ± 4†‡ |
| L194S/T254S | 280.0 ± 23.9 | ND | 57 ± 4† |
| L194A/T254S | 254.2 ± 50.3 | ND | 82 ± 3† |
Inactivation half-lives were determined by measuring residual APC activity after incubation in plasma as described in Materials and Methods. Clotting times in the aPTT assay were derived by using 0.25 μg/ml (except for Y302Q as noted), the concentration at which wt APC approximately doubled the clotting time relative to the no-APC buffer control (control clotting time = 38 ± 5 sec). Data are the average of three independent determinations performed in triplicate ± SD except as noted.
ND, not determined.
Results are the average of one determination performed in triplicate.
The concentration of Y302Q was 0.19 μg/ml.
Figure 2.
Inhibition of APC derivatives in citrated, pooled human plasma in the absence (A) or presence (B) of 10 units/ml unfractionated heparin. Each purified APC (20 nM each) was incubated in citrated human plasma, and residual activity was measured at various times as described in Materials and Methods by using the synthetic chromogenic substrate S2366. The data shown are the average of triplicate measurements ± SD.
To determine the contribution from PCI in the inhibition of Leu-194 APC derivatives in human plasma, additional experiments were performed in the presence of heparin (Fig. 2B). Unfractionated heparin is known to accelerate PCI (but not α1-AT) inhibition of APC by a template mechanism (40) and exhibits a bell-shaped dependence on the heparin concentration. For these analyses, the heparin concentration that provided maximal stimulation of inhibition was determined empirically to be 10 units/ml (data not shown). Under these conditions, the Leu-194 derivatives exhibited ≈3- to 4-fold increased half-lives (Table 1) compared with wt APC, demonstrating that the substitutions at Leu-194 affect the ability of APC to recognize PCI.
Importantly, engineered SERPIN resistance was achieved without significantly impacting the activity of APC toward its natural substrates. The average clotting times measured in aPTT assays for variants are listed in Table 1. The results shown were derived by using a plasma concentration at which wt APC approximately doubled the clotting time relative to the no-APC buffer control. In particular, the singly substituted Leu-194 variants retained nearly all of wt APC anticoagulant activity in the aPTT assay. Consistent with the retention of nearly complete anticoagulant activity, proteolytic inactivation of phospholipid-bound factor Va by the three Leu-194 variants were essentially identical to wt APC both in terms of the proteolytic pattern observed and the apparent kinetics of Va degradation (data not shown). In addition, kinetic analysis of amidolytic activity toward S2366 indicated similar intrinsic proteolytic activity for these variants relative to wt aPC: Km values for wt, L194S, L194A, and L194T were 0.63, 0.44, 0.48, and 0.66 mM, respectively, and the kcat values were 85, 78, 99, and 80 sec−1, respectively. Further detailed characterization, therefore, focused on the Leu-194 variants, because substitutions at this position yielded the largest apparent effects on SERPIN inhibition.
Inhibition Kinetics for Purified SERPINs.
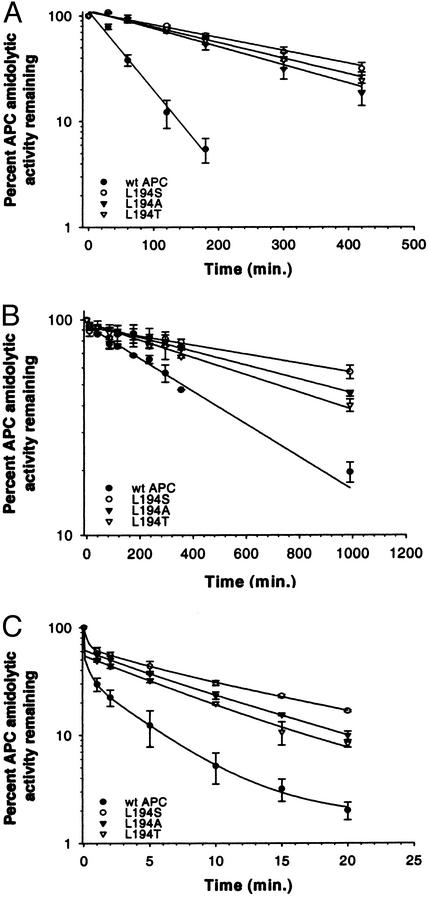
To assess more quantitatively the effects on specificity with respect to SERPIN recognition, studies were performed with purified α1-AT (Fig. 3A), PCI (Fig. 3B), and PCI in the presence of 10 units/ml heparin (Fig. 3C). The three Leu-194 variants were almost completely resistant to inhibition by purified α1-AT, with L194S exhibiting the greatest resistance. The inactivation half-lives, which were determined in the presence of the near-physiological concentration of 50 μΜ α1-AT, and second-order rate constants (k2) for inhibition, determined by measuring apparent first-order rates as a function of [α1-AT], are summarized in Table 2. The k2 calculated for inhibition of wt APC by α1-AT was 4.2 ± 0.5 M−1⋅sec−1 and is similar to that reported by Shen et al. (41). The L194S substitution resulted in a 5-fold reduction in the rate of α1-AT inhibition. The L194A and L194T substitutions had similar effects, with ≈4-fold lower k2 values than wt aPC.
Figure 3.
Inhibition of APC derivatives by purified SERPINs α1-AT (A), PCI (B), and PCI + heparin (C). APCs (20 nM) were incubated in AB/BSA containing α1-AT (A), 200 nM PCI (B), or 200 nM PCI and 10 units/ml heparin (C). Residual APC activity was determined at various times by measuring APC amidolytic activity as described in Materials and Methods. The data shown are the average of triplicate measurements ± SD.
Table 2.
Kinetic parameters for the inhibition of APC derivatives by purified SERPINs
| Variant | α1-AT k2, M−1⋅sec−1 | t1/2, min (50 μM α1-AT) | t1/2, min (200 nM PCI) |
|---|---|---|---|
| wtAPC | 4.2 ± 0.5 | 46 ± 8 | 394 ± 27 |
| L194S | 0.8 ± 0.1 | 247 ± 26 | 1,426 ± 194 |
| L194A | 1.1 ± 0.0 | 174 ± 24 | 929 ± 52 |
| L194T | 1.0 ± 0.3 | 203 ± 24 | 745 ± 39 |
Half-lives and rate constants derived from experiments with purified α1-AT and PCI were determined as described in Materials and Methods. Results are the average of three independent determinations, each performed in triplicate.
Similarly, Leu-194 variants were also resistant to inactivation by purified PCI (Fig. 3B), although not as dramatically as was their interaction with α1-AT. The three Leu-194 variants had comparable and extended half-lives with respect to inactivation by PCI in the absence of heparin, exhibiting a 3- to 4-fold increase compared with wt APC, with L194S being the most resistant (Table 2). When 10 units/ml heparin was added to the reaction mixtures, the inhibition rates were accelerated ≈20- to 40-fold (Fig. 3C). Inhibition kinetic constants in the presence of heparin were not determined because of the rate of inhibition; e.g., the residual wt APC amidolytic activity was only ≈30% after the first 30 sec of incubation. Nonetheless, the variants all exhibited similar behavior with respect to inhibition by PCI whether stimulated by heparin or not, further supporting the conclusion that changes are a result of proteolytic specificity changes and not due to some other unintended structural change.
PK Behavior.
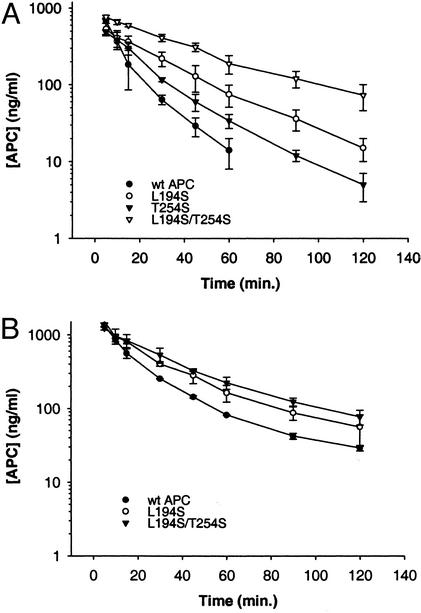
The PK behavior of the variants was examined in both rabbits and cynomolgus monkeys (Fig. 4) to determine the in vivo impact of resistance to SERPIN inhibition. In these species, the ranking of variants in plasma-inactivation experiments mirrored the ranking observed in human plasma, suggesting that SERPIN inhibition of human aPC in these species should be an adequate representation of what might be expected in humans. For example, the half-lives in rabbit plasma were 8 min for wt APC and 63 min for L194S, and in monkey plasma the half-lives were 73 and 269 min for wt APC and L194S, respectively. The increase in in vitro plasma half-lives translated to their in vivo PK behavior. In both species the variant APCs exhibited increases in exposure (area under the curve, ng⋅hr/ml) relative to wt-APC after bolus administration. In rabbits (Fig. 4A), relative to wt APC (114 ng⋅hr/ml), the average area under the curve increased ≈2-fold for the L194S variant (250 ng⋅hr/ml) and ≈5-fold with the L194S/T254S variant (578 ng⋅hr/ml). Similar effects were observed in cynomolgus monkeys (Fig. 4B) where the L194S variant increased exposure by ≈1.5-fold (627 ng⋅hr/ml), and the L194S/T254S variant (883 ng⋅hr/ml) approximately doubled the area under the curve compared with wt APC (430 ng⋅hr/ml). It was interesting to note that in rabbits the elimination half-life also increased for the variants (wt APC t1/2 was 12 min, whereas t1/2 measured for L194S and L194S/T254S was 23 and 36 min, respectively). Importantly, the ranking that had been observed for the variants in vitro was absolutely retained in vivo, confirming the primary role of SERPINS in regulating circulating APC levels in vivo.
Figure 4.
PKs in rabbit (A) and cynomolgus monkey (B). wt APC and variants were administered intravenously (100 μg/kg). Plasma levels of APC were determined by using an enzyme-capture assay as described in Materials and Methods. The data shown are the average ± SD for three rabbits (A) and two monkeys (B).
Notably, the magnitude of change relative to wt APC in vitro did not fully translate in vivo; e.g., the relative inactivation rates in vitro in rabbit plasma for L194S was ≈8-fold slower than wt APC, whereas the in vivo exposure increased only ≈2-fold. Similarly, for in vitro inhibition studies performed with cynomolgus monkey plasma, an ≈4-fold half-life extension was measured for L194S compared with wt APC, whereas the analogous comparison of PK profiles suggested smaller gains in exposure for L194S. These results indicate that there are other clearance mechanisms in vivo that presumably become more dominant as SERPIN-mediated clearance is reduced, as would be expected for a complex glycoprotein such as PC.
Discussion
Human APC is an attractive therapeutic for treatment of DIC, septic shock, or other thromboembolic conditions due to its anticoagulant and antiinflammatory properties. In fact, APC infusions have been administered successfully in cases of PC deficiency (42) and in the treatment of meningococcemia (43), DIC (44), and severe sepsis (8). The use of a SERPIN-resistant APC therapy could enhance its utility further, especially in disease states characterized by elevated levels of the SERPINs. In DIC, for example, a significant proportion of the total PC is found in complexes with α1-AT and PCI (16). The potential for the therapeutic use of APC to treat DIC, septic shock, and other thromboembolic conditions is complicated by the occurrence of a virtually inexhaustible supply of α1-AT (2.5 mg/ml in plasma) (15, 16). The other major plasma inhibitor of APC, PCI, is present at only 5 μg/ml and therefore is probably not as significant an obstacle to APC clinical therapy (45).
Molecular engineering of APC to ameliorate its inactivation by α1-AT without adversely impacting anticoagulant activity could present a molecule with better therapeutic potential by providing an entity that would be resistant to inactivation in disease states with elevated levels of SERPINs. The present data indicate that it indeed is possible to accomplish this. In particular, we have identified mutations in APC that have very large effects on SERPIN recognition but minor effects on natural substrate recognition or intrinsic proteolytic activity. The observation that most of the designed mutations achieved the intended result of prolonged activity in plasma suggests that the structural models created were sufficiently accurate. The only exception to this was the F316N substitution; however, a closer examination of the structural models indicates that this side chain doesn't form contacts with the substrate sequences that are as close or extensive as the side chains of Leu-194 or Ala-195, presumably explaining the properties of this variant. Interestingly, although the structure of the active site of APC can tolerate these mutations and still retain functional activity, it is clear that some residues are more important for maintaining this activity than others, e.g., the loss of significant anticoagulant activity resulting from the Y302Q substitution.
Two published accounts describe active-site mutations in APC that were created to understand the proteolytic specificity of APC: T254Y (23) and E357Q (22). Both substitutions were chosen based on their homologous location in other serine proteases, where these positions have also been examined. Although both mutations resulted in a 2-fold increased activity of APC toward inactivation of factor Va, they also resulted in ≈100- to 300-fold increased inhibition by SERPINs (23). Interestingly, changing both residues simultaneously altered the specificity of APC for small chromogenic substrates, but did not alter the specificity for the natural substrates of APC. In contrast, the amino acid substitutions we report were designed with the intent to retain biologic activity while impairing SERPIN recognition. Consistent with the results shown here, these studies indicated that small changes in the active site could lead to large changes in the inactivation by SERPINs, with little to no change in macromolecular specificity that might result in loss of function. In the case of APC, which has a fairly broad specificity with respect to its natural substrates, the specificity can be narrowed in a way such that nearly full biological activity is retained.
Our approach for altering SERPIN inhibition is somewhat different from examples that had similar intentions. For example, another coagulation protease with therapeutic utility, tissue-type plasminogen activator, has also been engineered to reduce SERPIN inactivation to improve its PK profile. In this case a 4-aa stretch in the serine-protease domain, KHRR (amino acids 296–299), was replaced with alanine at all positions and resulted in a substantial resistance to inhibition by the SERPIN plasminogen activator inhibitor-1 (46–50). Work with APC has demonstrated that loops within the protease domain, when substituted with the corresponding loops from bovine PC, resulted in SERPIN resistance (45). In these cases, the residues being modified are more likely to affect the surface interface in the protease–SERPIN complex. Importantly, we achieved substantial gains in plasma half-life and enhancements in in vivo PK profiles via single amino acid substitutions, which are less likely to be immunogenic in comparison to the other examples where multiple amino acid substitutions were used. In the case of APC, these single amino acid substitutions provided improvements in resistance to inactivation that were substantially better than the swapping of loops, suggesting that this approach to alter proteolytic specificity is ultimately much more efficient in engineering serine proteases for therapeutic purposes.
Acknowledgments
We gratefully acknowledge Dr. Faming Zhang and Dr. Rick Loncharich for assistance with the generation of the structural models and Dr. Uma Kuchibhotla for critically reviewing the manuscript. Eli Lilly and Company funded this research.
Abbreviations
- PC
protein C
- APC
activated PC
- SERPIN
serine protease inhibitor
- PCI
PC inhibitor
- α1-AT
α1-antitrypsin
- PK
pharmacokinetic
- DIC
disseminated intravascular coagulation
- S2366
l-pyroglutamyl-l-prolyl-l-arginine-p-nitroanilide
- wt
wild type
- aPTT
activated partial thromboplastin time
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Esmon C T. Arterioscler Thromb. 1992;12:135–145. doi: 10.1161/01.atv.12.2.135. [DOI] [PubMed] [Google Scholar]
- 2.Esmon C T, Ding W, Yasuhiro K, Gu J M, Ferrell G, Regan L M, Stearns-Kurosawa D J, Kurosawa S, Mather T, Laszik Z, Esmon N L. Thromb Haemostasis. 1997;78:70–74. [PubMed] [Google Scholar]
- 3.Yan S B, Grinnell B W. Annu Rep Med Chem. 1994;11:103–112. [Google Scholar]
- 4.Branson H E, Katz J, Marble R, Griffin J H. Lancet. 1983;2:1165–1168. doi: 10.1016/s0140-6736(83)91216-3. [DOI] [PubMed] [Google Scholar]
- 5.Seligsohn U, Berger A, Abend M, Rubin L, Attias D, Zivelin A, Rapaport S I. N Engl J Med. 1984;310:559–562. doi: 10.1056/NEJM198403013100904. [DOI] [PubMed] [Google Scholar]
- 6.Broekmans A W, Veltkamp J J, Bertina R M. N Engl J Med. 1983;309:340–344. doi: 10.1056/NEJM198308113090604. [DOI] [PubMed] [Google Scholar]
- 7.Joyce D E, Gelbert L, Ciaccia A, DeHoff B, Grinnell B W. J Biol Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 8.Bernard G R, Vincent J L, Laterre P F, LaRosa S P, Dhainaut J F, Lopez-Rodriguez A, Steingrub J S, Garber G E, Helterbrand J D, et al. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 9.Hermans J M, Stone S R. Biochem J. 1993;295:239–245. doi: 10.1042/bj2950239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heeb M J, Griffin J H. J Biol Chem. 1988;263:11613–11616. [PubMed] [Google Scholar]
- 11.Suzuki K, Nishioka J, Hashimoto S. J Biol Chem. 1983;258:163–168. [PubMed] [Google Scholar]
- 12.Whisstock J, Skinner R, Lesk A M. Trends Biochem Sci. 1998;23:63–67. doi: 10.1016/s0968-0004(97)01172-9. [DOI] [PubMed] [Google Scholar]
- 13.Loebermann H, Tokuoka R, Deisenhofer J, Huber R. J Mol Biol. 1984;177:531–557. [PubMed] [Google Scholar]
- 14.Okajima K, Koga S, Kaji M, Inoue M, Nakagaki T, Funatsu A, Okabe H, Takatsuki K, Aoki N. Thromb Haemostasis. 1990;63:48–53. [PubMed] [Google Scholar]
- 15.Morgan K, Kalsheker N A. Int J Biochem Cell Biol. 1997;29:1501–1511. doi: 10.1016/s1357-2725(97)00118-0. [DOI] [PubMed] [Google Scholar]
- 16.Scully M F, Toh C H, Hoogendoorn H, Manuel R P, Nesheim M E, Solymoss S, Giles A R. Thromb Haemostasis. 1993;69:448–453. [PubMed] [Google Scholar]
- 17.Somayajulu G L, Reddy P P. Indian J Pathol Microbiol. 1996;39:271–275. [PubMed] [Google Scholar]
- 18.Perona J J, Craik C S. Protein Sci. 1995;4:337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perona J J, Craik C S. J Biol Chem. 1997;272:29987–29990. doi: 10.1074/jbc.272.48.29987. [DOI] [PubMed] [Google Scholar]
- 20.Craik C S, Largman C, Fletcher T, Roczniak S, Barr P J, Fletterick R, Rutter W J. Science. 1985;228:291–297. doi: 10.1126/science.3838593. [DOI] [PubMed] [Google Scholar]
- 21.Harris J L, Craik C S. Curr Opin Chem Biol. 1998;2:127–132. doi: 10.1016/s1367-5931(98)80044-6. [DOI] [PubMed] [Google Scholar]
- 22.Rezaie A R, Esmon C T. Eur J Biochem. 1996;242:477–484. doi: 10.1111/j.1432-1033.1996.477rr.x. [DOI] [PubMed] [Google Scholar]
- 23.Rezaie A R. J Biol Chem. 1996;271:23807–23814. doi: 10.1074/jbc.271.39.23807. [DOI] [PubMed] [Google Scholar]
- 24.Mather T, Oganessyan V, Hof P, Huber R, Foundling S, Esmon C, Bode W. EMBO J. 1996;15:6822–6831. [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu X, Padmanabhan K P, Carperos V E, Tulinsky A, Kline T, Maraganore J M, Fenton J W, II. Biochemistry. 1992;31:11689–11697. doi: 10.1021/bi00162a004. [DOI] [PubMed] [Google Scholar]
- 26.Brooks B R, Bruccoleri R E, Olafson B D, States D J, Swaminathan S, Karplus M. J Comput Chem. 1983;4:187–217. [Google Scholar]
- 27.Grinnell B W, Berg D T, Walls J, Yan S B. Bio/Technology. 1987;5:1189–1192. [Google Scholar]
- 28.Berg D T, McClure D B, Grinnell B W. Nucleic Acids Res. 1992;20:5485–5486. doi: 10.1093/nar/20.20.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg D T, McClure D B, Grinnell B W. BioTechniques. 1993;14:972–978. [PubMed] [Google Scholar]
- 30.Jackson R J, Howell M T, Kaminski A. Trends Biochem Sci. 1990;15:477–483. doi: 10.1016/0968-0004(90)90302-r. [DOI] [PubMed] [Google Scholar]
- 31.Cormack B P, Valdivia R H, Falkow S. Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 32.Desai M A, Burnett J P, Mayne N G, Schoepp D D. Br J Pharmacol. 1996;118:1558–1564. doi: 10.1111/j.1476-5381.1996.tb15574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinnell B W, Walls J D, Gerlitz B. J Biol Chem. 1991;266:9778–9785. [PubMed] [Google Scholar]
- 34.Gerlitz B, Grinnell B W. J Biol Chem. 1996;271:22285–22288. doi: 10.1074/jbc.271.37.22285. [DOI] [PubMed] [Google Scholar]
- 35.Laemmli U K. Nature. 1970;27:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Kalafatis M, Rand M D, Mann K G. J Biol Chem. 1994;269:31869–31880. [PubMed] [Google Scholar]
- 37.Heeb M J, Bischoff R, Courtney M, Griffin J H. J Biol Chem. 1990;265:2365–2369. [PubMed] [Google Scholar]
- 38.Gruber A, Griffin J H. Blood. 1992;79:2340–2348. [PubMed] [Google Scholar]
- 39.Schechter I, Berger A. Biochem Biophys Res Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 40.Pratt C W, Church F C. Blood Coagul Fibrinolysis. 1993;4:479–490. doi: 10.1097/00001721-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Shen L, Dahlback B, Villoutreix B O. Biochemistry. 2000;39:2853–2860. doi: 10.1021/bi992357p. [DOI] [PubMed] [Google Scholar]
- 42.Dreyfus M, Magny J F, Bridey F, Schwarz H P, Planche C, Dehan M, Tchernia G. N Engl J Med. 1991;325:1565–1568. doi: 10.1056/NEJM199111283252207. [DOI] [PubMed] [Google Scholar]
- 43.White B, Livingstone W, Murphy C, Hodgson A, Rafferty M, Smith O P. Blood. 2000;96:3719–3724. [PubMed] [Google Scholar]
- 44.Okajima K, Imamura H, Koga S, Inoue M, Takatsuki K, Aoki N. Am J Hematol. 1990;33:277–278. doi: 10.1002/ajh.2830330413. [DOI] [PubMed] [Google Scholar]
- 45.Holly R D, Foster D C. Biochemistry. 1994;33:1876–1880. doi: 10.1021/bi00173a034. [DOI] [PubMed] [Google Scholar]
- 46.Paoni N F, Keyt B A, Refino C J, Chow A M, Nguyen H V, Berleau L T, Badillo J, Pena L C, Brady K, Wurm F M, et al. Thromb Haemostasis. 1993;70:307–312. [PubMed] [Google Scholar]
- 47.Refino C J, Paoni N F, Keyt B A, Pater C S, Badillo J M, Wurm F M, Ogez J, Bennett W F. Thromb Haemostasis. 1993;70:313–319. [PubMed] [Google Scholar]
- 48.Keyt B A, Paoni N F, Refino C J, Berleau L, Nguyen H, Chow A, Lai J, Pena L, Pater C, Ogez J, et al. Proc Natl Acad Sci USA. 1994;91:3670–3674. doi: 10.1073/pnas.91.9.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madison E L, Goldsmith E J, Gerard R D, Gething M J, Sambrook J F. Nature. 1989;339:721–724. doi: 10.1038/339721a0. [DOI] [PubMed] [Google Scholar]
- 50.Madison E L, Goldsmith E J, Gerard R D, Gething M J, Sambrook J F, Bassel-Duby R S. Proc Natl Acad Sci USA. 1990;87:3530–3533. doi: 10.1073/pnas.87.9.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]