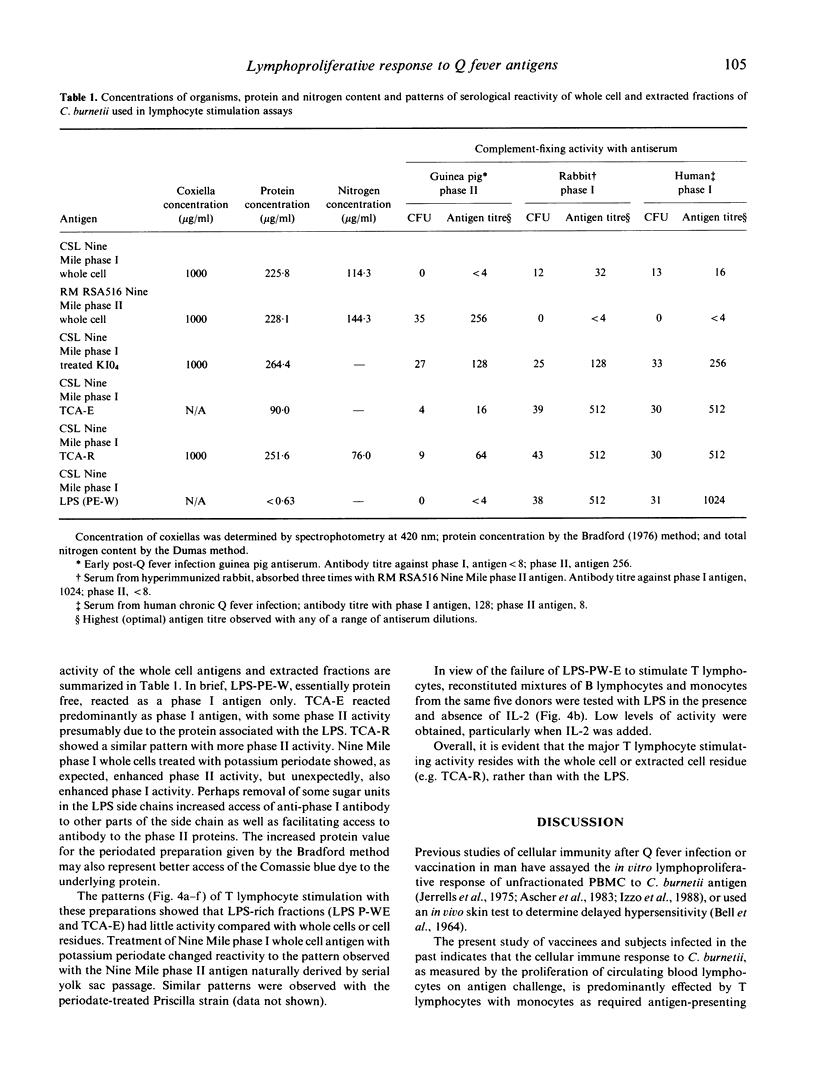
Abstract
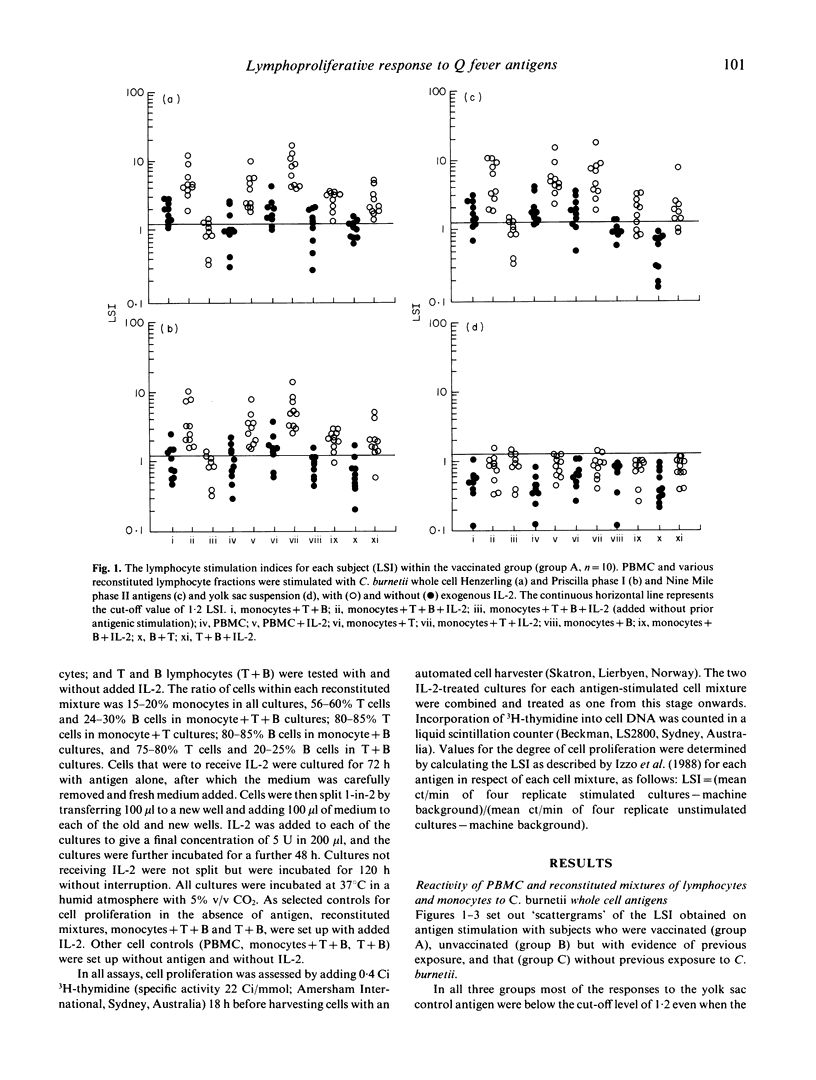
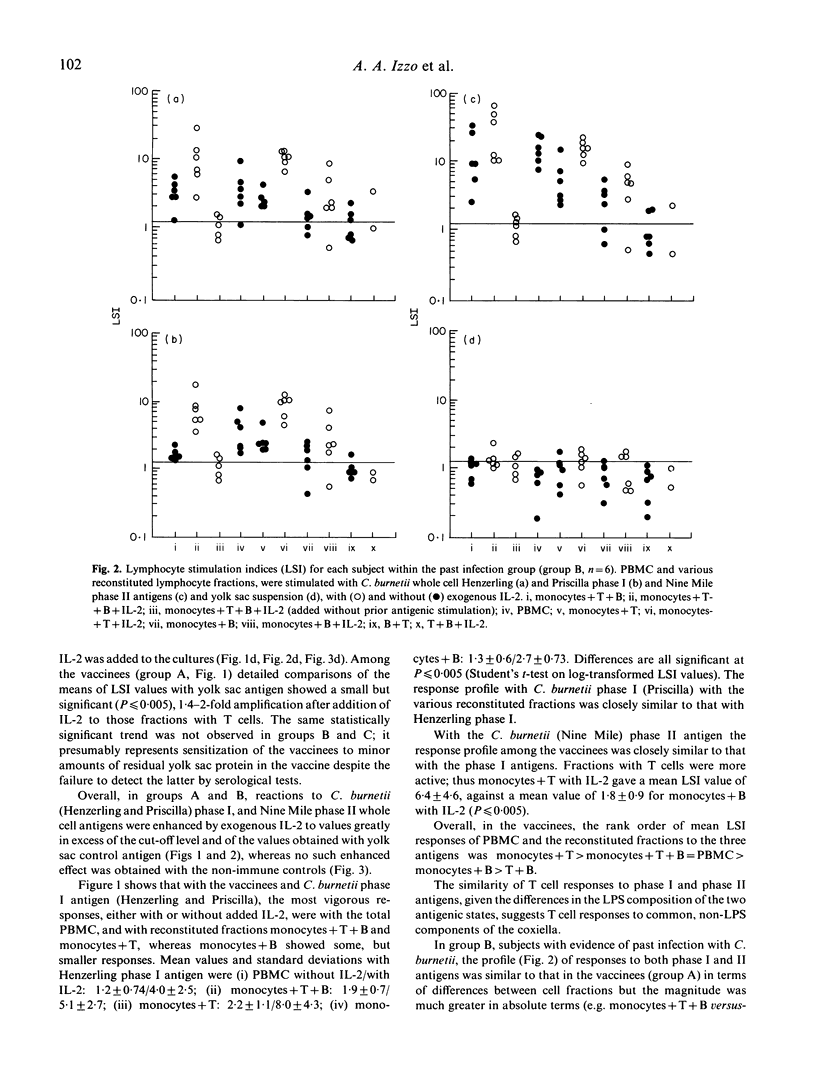
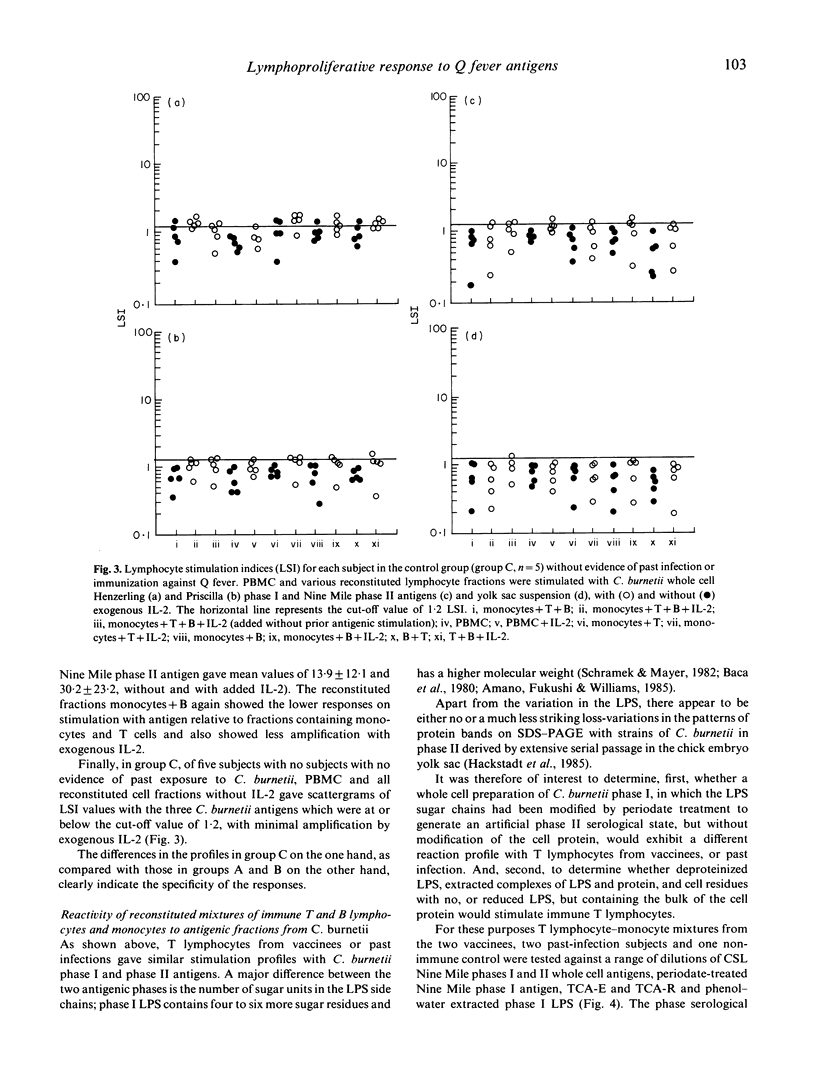
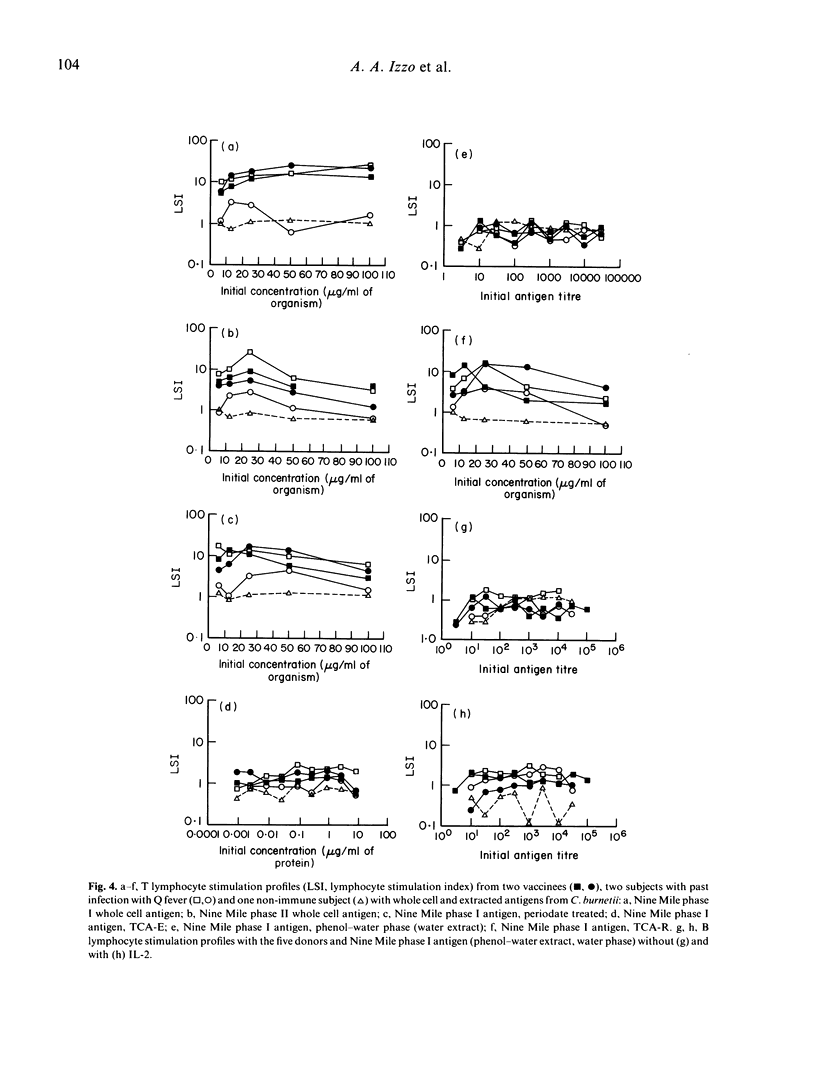
Vaccination with an inactivated, whole cell, Q fever vaccine (Q-vax) induces lasting antibody conversion and a positive delayed-type hypersensitivity (DTH) skin reaction in about 60% of recipients but a long-lasting positive lymphoproliferative or mitogenic response to C. burnetii antigens with peripheral blood mononuclear cells (PBMC) in 85-95% of subjects. Analysis of the lymphoproliferative response to C. burnetii antigens has now been made by fractionation-reconstitution experiments with PBMC from vaccines, from past infections, and from healthy controls. The major contributor to the response in immune subjects proved to be the T lymphocyte. T cells were stimulated by both the phase I and phase II antigens of two prototype strains of C. burnetii and responses were greatly amplified by addition of IL-2. Similar T lymphocyte stimulation profiles were obtained with the 'Priscilla' strain of C. burnetii which represents a different biotype of Coxiella isolated from Q fever endocarditis; Q-vax is therefore likely to protect against endocarditis strains. Fractionation-reconstitution experiments with T and B cells from vaccines and subjects infected in the past, using various antigenic or haptenic fractions from C. burnetii indicate that protein, non-lipopolysaccharide components of the organism are responsible for the mitogenic response of immune T cells. However, the role of the lipopolysaccharide in the protective immunogen has still to be defined.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABINANTI F. R., MARMION B. P. Protective or neutralizing antibody in Q fever. Am J Hyg. 1957 Sep;66(2):173–195. doi: 10.1093/oxfordjournals.aje.a119894. [DOI] [PubMed] [Google Scholar]
- ANACKER R. L., HASKINS W. T., LACKMAN D. B., RIBI E., PICKENS E. G. CONVERSION OF THE PHASE I ANTIGEN OF COXIELLA BURNETII TO HAPTEN BY PHENOL TREATMENT. J Bacteriol. 1963 May;85:1165–1170. doi: 10.1128/jb.85.5.1165-1170.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sumidaie A. M., Jones D. L., Young H. L. Characterisation of the under-agarose method for quantifying migration of highly purified human monocytes. J Immunol Methods. 1984 Dec 14;75(1):129–140. doi: 10.1016/0022-1759(84)90232-1. [DOI] [PubMed] [Google Scholar]
- Amano K., Fukushi K., Williams J. C. Electron microscopic studies of lipopolysaccharides from phase I and phase II Coxiella burnetii. J Gen Microbiol. 1985 Nov;131(11):3127–3130. doi: 10.1099/00221287-131-11-3127. [DOI] [PubMed] [Google Scholar]
- Ascher M. S., Berman M. A., Ruppanner R. Initial clinical and immunologic evaluation of a new phase I Q fever vaccine and skin test in humans. J Infect Dis. 1983 Aug;148(2):214–222. doi: 10.1093/infdis/148.2.214. [DOI] [PubMed] [Google Scholar]
- BELL J. F., LUOTO L., CASEY M., LACKMAN D. B. SEROLOGIC AND SKIN-TEST RESPONSE AFTER Q FEVER VACCINATION BY THE INTRACUTANEOUS ROUTE. J Immunol. 1964 Sep;93:403–408. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brezina R., Pospísil V., Schramek S. Study of the antigenic structure of Coxiella burneti. VII. Properties of phenol-extracted phase I antigenic component. Acta Virol. 1970 Jul;14(4):295–301. [PubMed] [Google Scholar]
- Brezina R., Pospísil V. Study of the antigenic structure of Coxiella burneti. 8. Immunogenicity of phenol-extracted phase I antigenic component. Acta Virol. 1970 Jul;14(4):302–306. [PubMed] [Google Scholar]
- Brooks D. L., Ermel R. W., Franti C. E., Ruppanner R., Behymer D. E., Williams J. C., Stephenson E. H., Stephenson J. C. Q fever vaccination of sheep: challenge of immunity in ewes. Am J Vet Res. 1986 Jun;47(6):1235–1238. [PubMed] [Google Scholar]
- Crowle A. J. Immunization against tuberculosis: what kind of vaccine? Infect Immun. 1988 Nov;56(11):2769–2773. doi: 10.1128/iai.56.11.2769-2773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- Hackstadt T. Antigenic variation in the phase I lipopolysaccharide of Coxiella burnetii isolates. Infect Immun. 1986 Apr;52(1):337–340. doi: 10.1128/iai.52.1.337-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Peacock M. G., Hitchcock P. J., Cole R. L. Lipopolysaccharide variation in Coxiella burnetti: intrastrain heterogeneity in structure and antigenicity. Infect Immun. 1985 May;48(2):359–365. doi: 10.1128/iai.48.2.359-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphres R. C., Hinrichs D. J. Role of antibody in Coxiella burnetii infection. Infect Immun. 1981 Feb;31(2):641–645. doi: 10.1128/iai.31.2.641-645.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A. A., Marmion B. P., Worswick D. A. Markers of cell-mediated immunity after vaccination with an inactivated, whole-cell Q fever vaccine. J Infect Dis. 1988 Apr;157(4):781–789. doi: 10.1093/infdis/157.4.781. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Mallavia L. P., Hinrichs D. J. Detection of long-term cellular immunity to Coxiella burneti as assayed by lymphocyte transformation. Infect Immun. 1975 Feb;11(2):280–286. doi: 10.1128/iai.11.2.280-286.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazár J., Brezina R., Kovácová E., Urvölgyi J. Testing in various systems of the neutralizing capacity of Q fever immune sera. Acta Virol. 1973 Jan;17(1):79–89. [PubMed] [Google Scholar]
- Kishimoto R. A., Rozmiarek H., Larson E. W. Experimental Q fever infection in congenitally athymic nude mice. Infect Immun. 1978 Oct;22(1):69–71. doi: 10.1128/iai.22.1.69-71.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukácová M., Brezina R., Schramek S., Pastorek J. Chemical composition of phase I Coxiella burnetii soluble antigen prepared by trichloroacetic acid extraction. Acta Virol. 1989 Jan;33(1):75–80. [PubMed] [Google Scholar]
- Marmion B. P., Ormsbee R. A., Kyrkou M., Wright J., Worswick D., Cameron S., Esterman A., Feery B., Collins W. Vaccine prophylaxis of abattoir-associated Q fever. Lancet. 1984 Dec 22;2(8417-8418):1411–1414. doi: 10.1016/s0140-6736(84)91617-9. [DOI] [PubMed] [Google Scholar]
- Moll H., Mitchell G. F., McConville M. J., Handman E. Evidence of T-cell recognition in mice of a purified lipophosphoglycan from Leishmania major. Infect Immun. 1989 Nov;57(11):3349–3356. doi: 10.1128/iai.57.11.3349-3356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos A., Hackstadt T. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect Immun. 1987 May;55(5):1144–1150. doi: 10.1128/iai.55.5.1144-1150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMSBEE R. A. A method of purifying Coxiella burnetii and other pathogenic Rickettsiae. J Immunol. 1962 Jan;88:100–108. [PubMed] [Google Scholar]
- ORMSBEE R. A., BELL E. J., LACKMAN D. B. Antigens of Coxiella burnetii. I. Extraction of antigens with non-aqueous organic solvents. J Immunol. 1962 Jun;88:741–749. [PubMed] [Google Scholar]
- ORMSBEE R. A., BELL E. J., LACKMAN D. B., TALLENT G. THE INFLUENCE OF PHASE ON THE PROTECTIVE POTENCY OF Q FEVER VACCINE. J Immunol. 1964 Mar;92:404–412. [PubMed] [Google Scholar]
- Paquet A., Jr, Rael E. D., Klassen D., Martinez I., Baca O. G. Mitogenic and protective activity associated with a lipopolysaccharide from Coxiella burnetii. Can J Microbiol. 1978 Dec;24(12):1616–1618. doi: 10.1139/m78-259. [DOI] [PubMed] [Google Scholar]
- Samuel J. E., Frazier M. E., Mallavia L. P. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect Immun. 1985 Sep;49(3):775–779. doi: 10.1128/iai.49.3.775-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramek S., Brezina R., Urvölgyi J. A new method of preparing diagnostic Q fever antigen. Acta Virol. 1972 Nov;16(6):487–492. [PubMed] [Google Scholar]
- Schramek S., Mayer H. Different sugar compositions of lipopolysaccharides isolated from phase I and pure phase II cells of Coxiella burnetii. Infect Immun. 1982 Oct;38(1):53–57. doi: 10.1128/iai.38.1.53-57.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer D. S., DeSanctis A. N., Beiler J. M. Preparation of highly purified concentrates of Coxiella burneti. Proc Soc Exp Biol Med. 1970 Dec;135(3):706–708. doi: 10.3181/00379727-135-35125. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Damrow T. A., Waag D. M., Amano K. Characterization of a phase I Coxiella burnetii chloroform-methanol residue vaccine that induces active immunity against Q fever in C57BL/10 ScN mice. Infect Immun. 1986 Mar;51(3):851–858. doi: 10.1128/iai.51.3.851-858.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Thomas L. A., Peacock M. G. Identification of phase-specific antigenic fractions of Coxiella burnetti by enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Dec;24(6):929–934. doi: 10.1128/jcm.24.6.929-934.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worswick D., Marmion B. P. Antibody responses in acute and chronic Q fever and in subjects vaccinated against Q fever. J Med Microbiol. 1985 Jun;19(3):281–296. doi: 10.1099/00222615-19-3-281. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


