Abstract
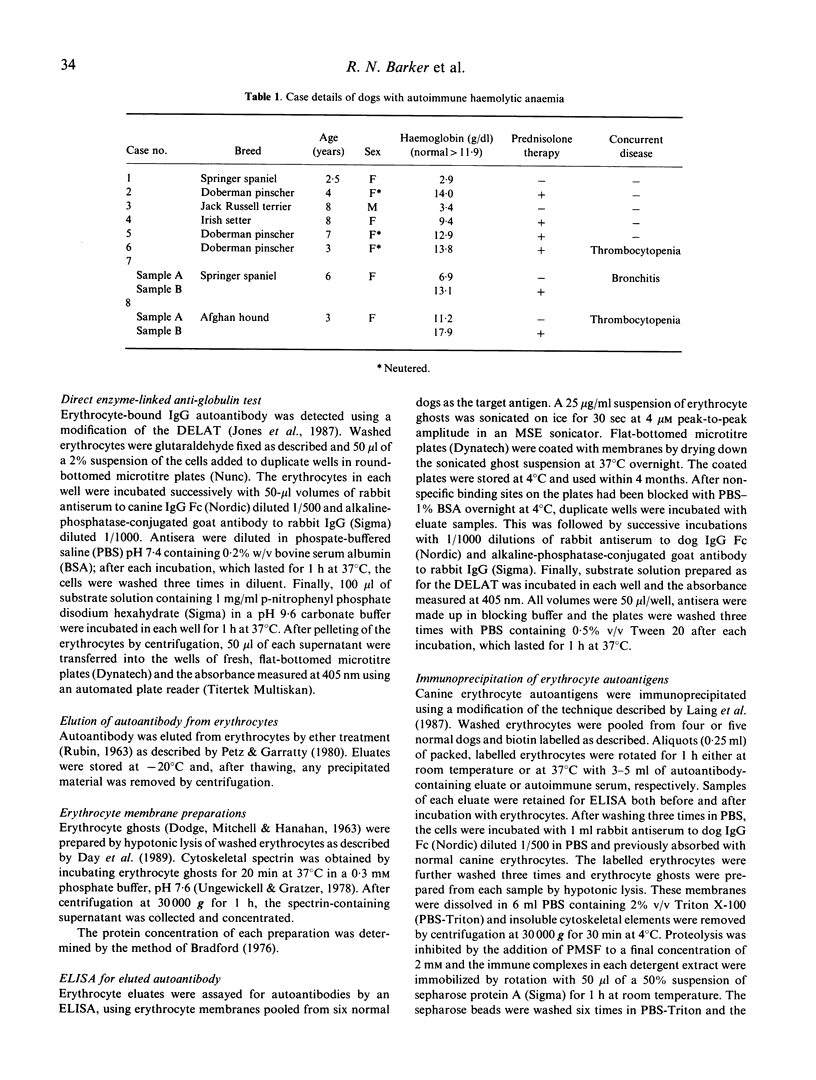
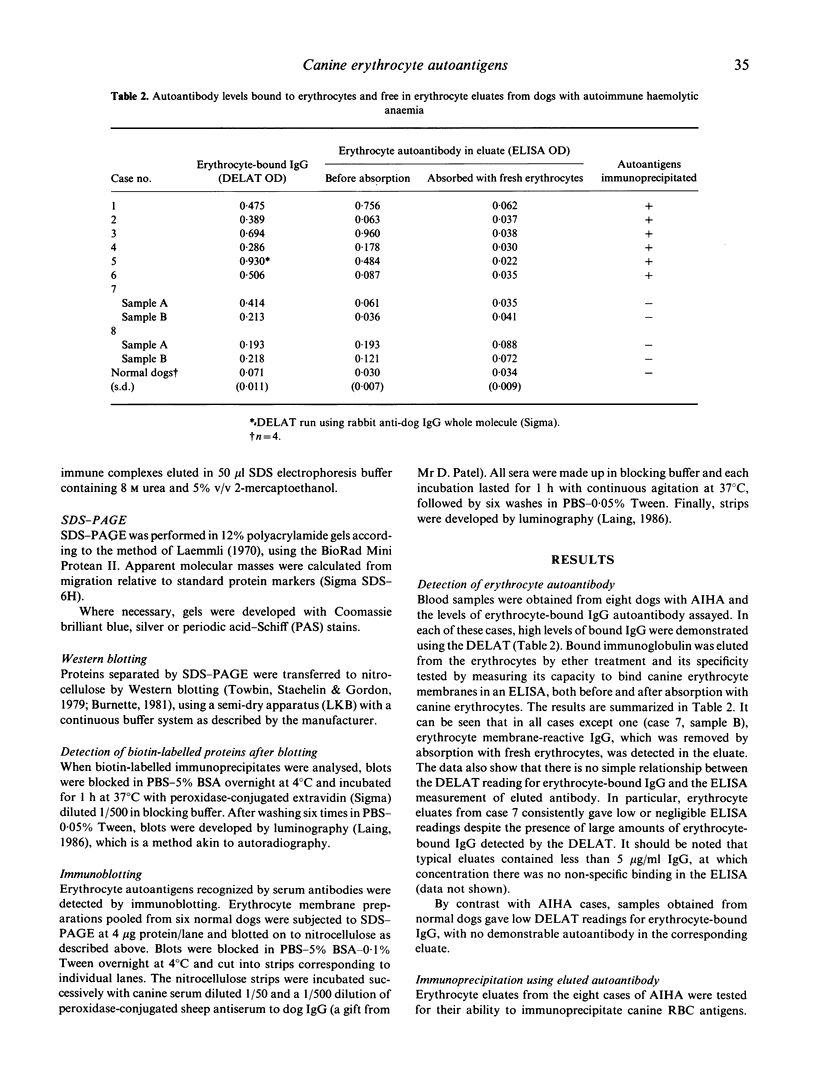
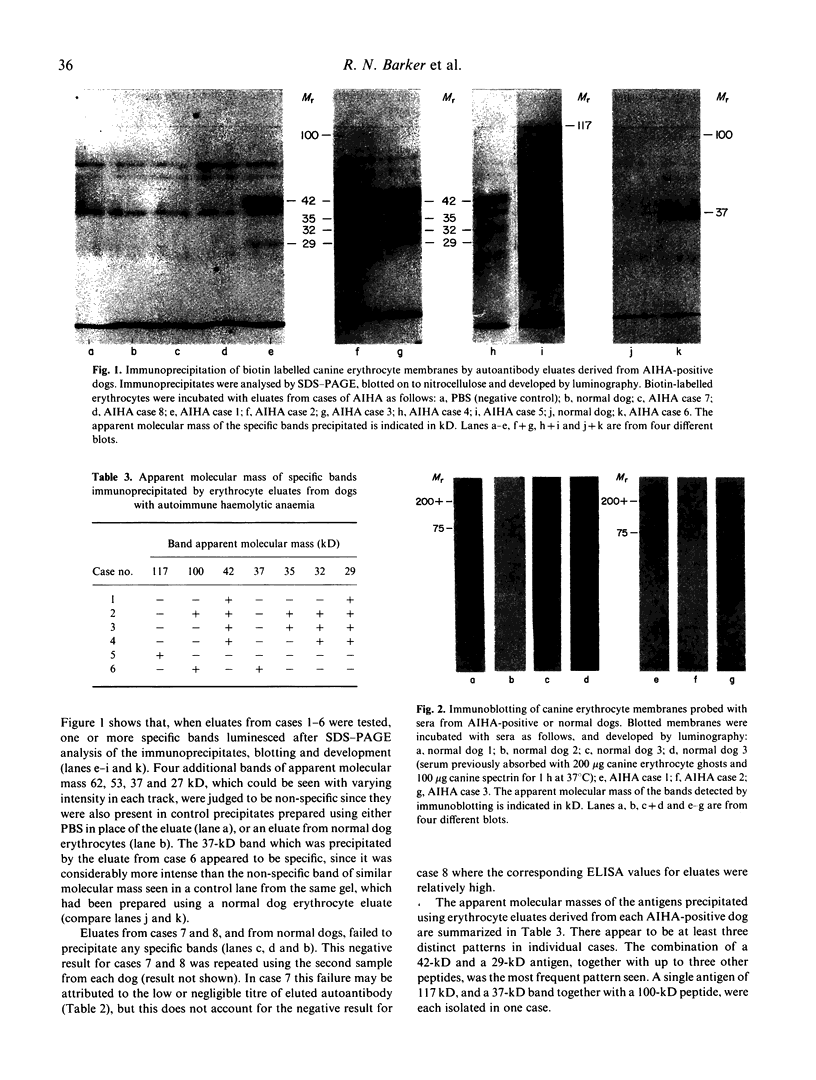
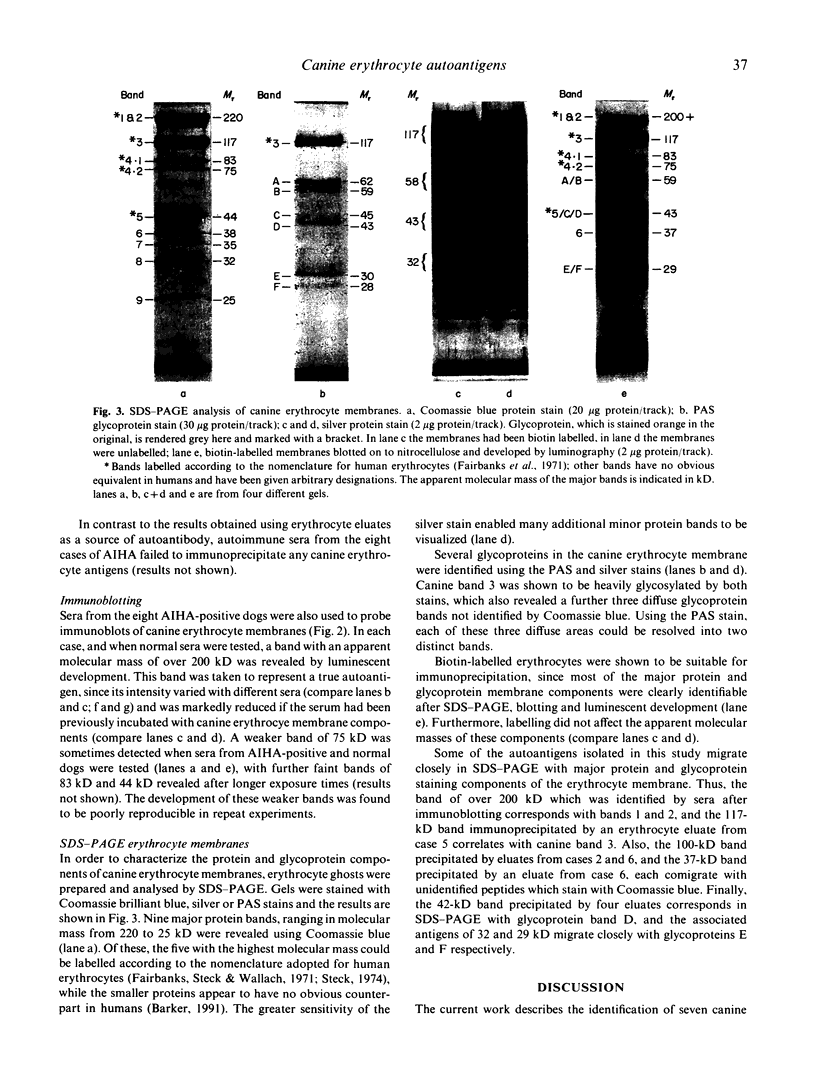
Autoantigens in canine autoimmune haemolytic anaemia (AIHA) were identified by immunoprecipitation using autoantibody eluted from the erythrocytes of affected dogs. At least three patterns of precipitated antigen were identified in six cases of AIHA. The most commonly precipitated antigen pattern was a combination of 42-kD and 29-kD peptides, associated with up to three other membrane components. These autoantigens may be canine glycophorins, which are of similar molecular mass, or may possibly represent an equivalent of the human Rhesus complex. An autoantigen identical in molecular mass to band 3, the erythrocyte anion channel protein, was precipitated in one case of AIHA, and unknown peptides of 37 kD and 100 kD were isolated by autoantibody from another dog. In one case, no antigens were precipitated by the eluted antibody, indicating that the autoantibody may have bound a non-protein membrane component such as phospholipid. Overall it is considered that the different patterns observed may reflect differences in the aetiology of the condition. In other studies, sera from dogs with AIHA failed to immunoprecipitate autoantigens, but were shown by immunoblotting to contain autoantibodies to proteins of the erythrocyte cytoskeleton. Such autoantibodies were also demonstrated in normal canine sera and it is suggested that they are unlikely to play a role in the pathogenesis of AIHA, but may be part of a normal clearance mechanism for damaged red blood cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballas S. K. Spectrin autoantibodies in normal human serum and in polyclonal blood grouping sera. Br J Haematol. 1989 Jan;71(1):137–139. doi: 10.1111/j.1365-2141.1989.tb06287.x. [DOI] [PubMed] [Google Scholar]
- Bowman H. S., Marsh W. L., Schumacher H. R., Oyen R., Reihart J. Auto anti-N immunohemolytic anemia in infectious mononucleosis. Am J Clin Pathol. 1974 Apr;61(4):465–472. doi: 10.1093/ajcp/61.4.465. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Campbell K. L., George J. W. Application of the enzyme-linked immunosorbent assay to detect canine erythrocyte antibodies. Am J Vet Res. 1984 Apr;45(4):747–750. [PubMed] [Google Scholar]
- Cohen D. W., Garratty G., Morel P., Petz L. D. Autoimmune hemolytic anemia associated with IgG auto anti-N. Transfusion. 1979 May-Jun;19(3):329–331. doi: 10.1046/j.1537-2995.1979.19379204217.x. [DOI] [PubMed] [Google Scholar]
- Cox K. O., Hardy S. J. Autoantibodies against mouse bromelain-modified RBC are specifically inhibited by a common membrane phospholipid, phosphatidylcholine. Immunology. 1985 Jun;55(2):263–269. [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Day M. J., Russell J., Kitwood A. J., Ponsford M., Elson C. J. Expression and regulation of erythrocyte auto-antibodies in mice following immunization with rat erythrocytes. Eur J Immunol. 1989 May;19(5):795–801. doi: 10.1002/eji.1830190503. [DOI] [PubMed] [Google Scholar]
- Dube V. E., House R. F., Jr, Moulds J., Polesky H. F. Hemolytic anemia caused by auto anti-N. Am J Clin Pathol. 1975 Jun;63(6):828–831. doi: 10.1093/ajcp/63.6.828. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Finne J. Identification of the blood-group ABH-active glycoprotein components of human erythrocyte membrane. Eur J Biochem. 1980 Feb;104(1):181–189. doi: 10.1111/j.1432-1033.1980.tb04414.x. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G. Molecular identification of the human Rho (D) antigen. FEBS Lett. 1982 Apr 5;140(1):93–97. doi: 10.1016/0014-5793(82)80528-0. [DOI] [PubMed] [Google Scholar]
- Hamaguchi H., Cleve H. Solubilization of human erythrocyte membrane glycoproteins and separation of the MN glycoprotein from a glycoprotein with I, S, and A activity. Biochim Biophys Acta. 1972 Sep 29;278(2):271–280. doi: 10.1016/0005-2795(72)90232-2. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Cox K. O. Mouse autoantibodies bind to a phospholipase-C-sensitive structure on red blood cells. Int Arch Allergy Appl Immunol. 1990;91(1):108–110. doi: 10.1159/000235098. [DOI] [PubMed] [Google Scholar]
- Hazeltine M., Rauch J., Danoff D., Esdaile J. M., Tannenbaum H. Antiphospholipid antibodies in systemic lupus erythematosus: evidence of an association with positive Coombs' and hypocomplementemia. J Rheumatol. 1988 Jan;15(1):80–86. [PubMed] [Google Scholar]
- Jones D. R., Darke P. G. Use of papain for the detection of incomplete erythrocyte autoantibodies in autoimmune haemolytic anaemia of the dog and cat. J Small Anim Pract. 1975 Apr;16(4):273–279. doi: 10.1111/j.1748-5827.1975.tb05744.x. [DOI] [PubMed] [Google Scholar]
- Jones D. R., Gruffydd-Jones T. J., Stokes C. R., Bourne F. J. Investigation into factors influencing performance of the canine antiglobulin test. Res Vet Sci. 1990 Jan;48(1):53–58. [PubMed] [Google Scholar]
- Jones D. R., Stokes C. R., Gruffydd-Jones T. J., Bourne F. J. An enzyme-linked antiglobulin test for the detection of erythrocyte-bound antibodies in canine autoimmune haemolytic anaemia. Vet Immunol Immunopathol. 1987 Sep;16(1-2):11–21. doi: 10.1016/0165-2427(87)90170-x. [DOI] [PubMed] [Google Scholar]
- Jones D. R. Use of an enzyme indirect antiglobulin test for the diagnosis of autoimmune haemolytic anaemia in the dog. Res Vet Sci. 1986 Sep;41(2):187–190. [PubMed] [Google Scholar]
- Karhi K. K., Gahmberg C. G. Identification of blood group A-active glycoproteins in the human erythrocyte membrane. Biochim Biophys Acta. 1980 Apr 25;622(2):344–354. doi: 10.1016/0005-2795(80)90046-x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi S. Phospholipid epitopes for mouse antibodies against bromelain-treated mouse erythrocytes. Immunology. 1987 Sep;62(1):11–16. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laing P., Appleby L. A., Culbert E. J., Elson C. J. Antigenicity of rat erythrocyte glycophorins. Immunol Lett. 1988 Jun;18(2):125–128. doi: 10.1016/0165-2478(88)90052-1. [DOI] [PubMed] [Google Scholar]
- Laing P. Luminescent visualization of antigens on blots. J Immunol Methods. 1986 Sep 27;92(2):161–165. doi: 10.1016/0022-1759(86)90161-4. [DOI] [PubMed] [Google Scholar]
- Laing P., Parkar B. A., Culbert E. J., Watt G. J., Elson C. J. Identification of rat erythrocyte antigens with a new non-radioactive immunoprecipitation technique. Scand J Immunol. 1987 Jun;25(6):613–620. doi: 10.1111/j.1365-3083.1987.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Lutz H. U., Wipf G. Naturally occurring autoantibodies to skeletal proteins from human red blood cells. J Immunol. 1982 Apr;128(4):1695–1699. [PubMed] [Google Scholar]
- Moore S., Green C. The identification of specific Rhesus-polypeptide-blood-group-ABH-active-glycoprotein complexes in the human red-cell membrane. Biochem J. 1987 Jun 15;244(3):735–741. doi: 10.1042/bj2440735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Woodrow C. F., McClelland D. B. Isolation of membrane components associated with human red cell antigens Rh(D), (c), (E) and Fy. Nature. 1982 Feb 11;295(5849):529–531. doi: 10.1038/295529a0. [DOI] [PubMed] [Google Scholar]
- Parker A. C., Willis G., Urbaniak S. J., Innes E. M. Autoimmune haemolytic anaemia with anti-A autoantibody. Br Med J. 1978 Jan 7;1(6104):26–26. doi: 10.1136/bmj.1.6104.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., Marshall-Clarke S. Induction of red cell autoantibodies in normal mice. Nat New Biol. 1973 Jun 13;243(128):213–214. doi: 10.1038/newbio243213a0. [DOI] [PubMed] [Google Scholar]
- RUBIN H. Antibody elution from red blood cells. J Clin Pathol. 1963 Jan;16:70–73. doi: 10.1136/jcp.16.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgwell K., Roberts S. J., Tanner M. J., Anstee D. J. Absence of two membrane proteins containing extracellular thiol groups in Rhnull human erythrocytes. Biochem J. 1983 Jul 1;213(1):267–269. doi: 10.1042/bj2130267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol R. J., Hewitt S. Autoimmune hemolysis: a critical review. Crit Rev Oncol Hematol. 1985;4(2):125–154. doi: 10.1016/s1040-8428(85)80013-5. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski I. O., Roberts P. L., Rosenfield R. E. Anti-A autoantibody with severe intravascular hemolysis. N Engl J Med. 1976 Apr 29;294(18):995–996. doi: 10.1056/NEJM197604292941807. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem. 1978 Aug 1;88(2):379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- Victoria E. J., Pierce S. W., Branks M. J., Masouredis S. P. IgG red blood cell autoantibodies in autoimmune hemolytic anemia bind to epitopes on red blood cell membrane band 3 glycoprotein. J Lab Clin Med. 1990 Jan;115(1):74–88. [PubMed] [Google Scholar]
- WEINER W., VOS G. H. SEROLOGY OF ACQUIRED HEMOLYTIC ANEMIAS. Blood. 1963 Nov;22:606–613. [PubMed] [Google Scholar]





