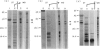Abstract
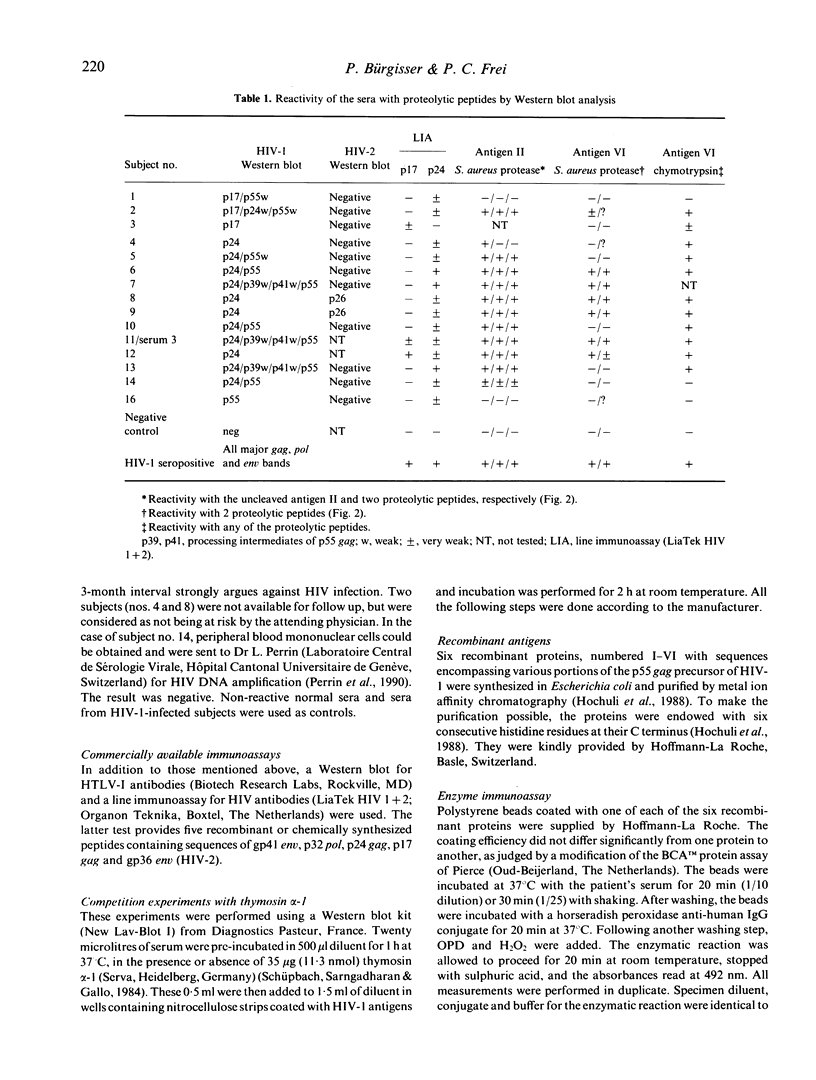
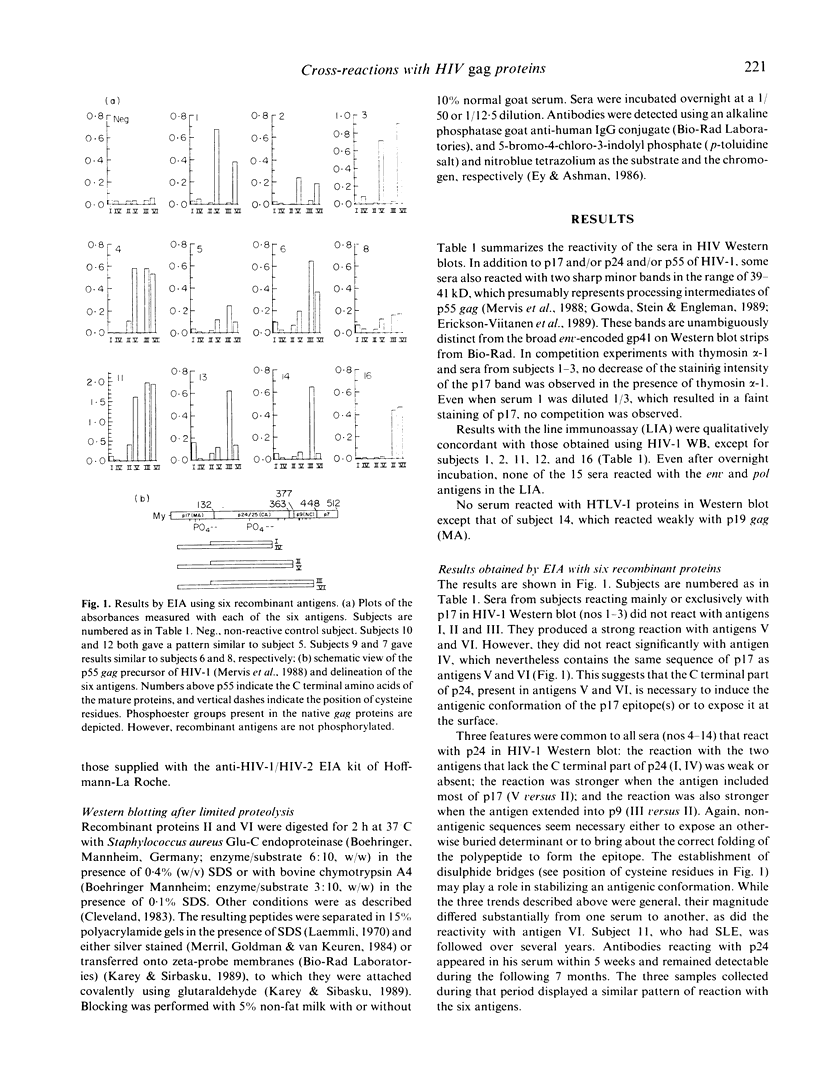
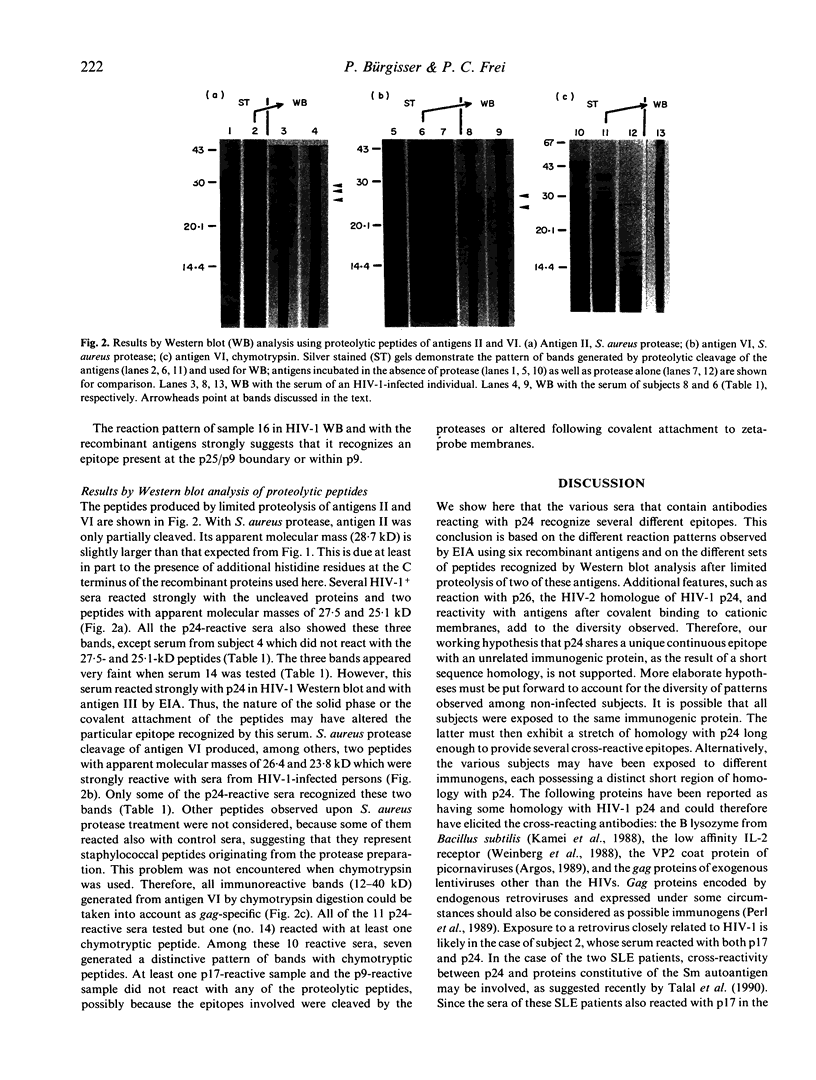
The use of serological tests for the diagnosis of HIV infection has revealed that some non-infected persons have antibodies that react with HIV-1 gag proteins. Here, the sera of three non-infected subjects reacting with p17 and 11 non-infected subjects reacting with p24 were investigated, using an enzyme immunoassay (EIA) with six recombinant gag antigens and Western blot analysis of proteolytic peptides of two of these gag antigens. The results indicate that whereas all p17-reactive sera could react with an unique epitope, individual p24-reactive sera recognize different epitopes. Investigations by EIA also demonstrated the role of sequences located far from the epitopes in making these epitopes accessible to the antibodies or in providing them with an antigenic conformation. In addition to the 14 subjects mentioned above, another subject was shown to have antibodies reacting with the p9 (NC) gag protein. Several proteins are known as having homology with HIV-1 gag proteins. Their possible role in eliciting cross-reactive antibodies is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P. A possible homology between immunodeficiency virus p24 core protein and picornaviral VP2 coat protein: prediction of HIV p24 antigenic sites. EMBO J. 1989 Mar;8(3):779–785. doi: 10.1002/j.1460-2075.1989.tb03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W. Peptide mapping in one dimension by limited proteolysis of sodium dodecyl sulfate-solubilized proteins. Methods Enzymol. 1983;96:222–229. doi: 10.1016/s0076-6879(83)96020-2. [DOI] [PubMed] [Google Scholar]
- Dock N. L., Lamberson H. V., Jr, O'Brien T. A., Tribe D. E., Alexander S. S., Poiesz B. J. Evaluation of atypical human immunodeficiency virus immunoblot reactivity in blood donors. Transfusion. 1988 Sep-Oct;28(5):412–418. doi: 10.1046/j.1537-2995.1988.28588337326.x. [DOI] [PubMed] [Google Scholar]
- Erickson-Viitanen S., Manfredi J., Viitanen P., Tribe D. E., Tritch R., Hutchison C. A., 3rd, Loeb D. D., Swanstrom R. Cleavage of HIV-1 gag polyprotein synthesized in vitro: sequential cleavage by the viral protease. AIDS Res Hum Retroviruses. 1989 Dec;5(6):577–591. doi: 10.1089/aid.1989.5.577. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Ashman L. K. The use of alkaline phosphatase-conjugated anti-immunoglobulin with immunoblots for determining the specificity of monoclonal antibodies to protein mixtures. Methods Enzymol. 1986;121:497–509. doi: 10.1016/0076-6879(86)21050-2. [DOI] [PubMed] [Google Scholar]
- Garry R. F., Fermin C. D., Hart D. J., Alexander S. S., Donehower L. A., Luo-Zhang H. Detection of a human intracisternal A-type retroviral particle antigenically related to HIV. Science. 1990 Nov 23;250(4984):1127–1129. doi: 10.1126/science.1701273. [DOI] [PubMed] [Google Scholar]
- Gowda S. D., Stein B. S., Engleman E. G. Identification of protein intermediates in the processing of the p55 HIV-1 gag precursor in cells infected with recombinant vaccinia virus. J Biol Chem. 1989 May 25;264(15):8459–8462. [PubMed] [Google Scholar]
- Guldner H. H., Netter H. J., Szostecki C., Jaeger E., Will H. Human anti-p68 autoantibodies recognize a common epitope of U1 RNA containing small nuclear ribonucleoprotein and influenza B virus. J Exp Med. 1990 Mar 1;171(3):819–829. doi: 10.1084/jem.171.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson S. L., Swack N. S., Ramirez M. T., Hausler W. J., Jr Investigation of atypical western blot (immunoblot) reactivity involving core proteins of human immunodeficiency virus type. J Clin Microbiol. 1989 May;27(5):932–937. doi: 10.1128/jcm.27.5.932-937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei K., Hara S., Ikenaka T., Murao S. Amino acid sequence of a lysozyme (B-enzyme) from Bacillus subtilis YT-25. J Biochem. 1988 Nov;104(5):832–836. doi: 10.1093/oxfordjournals.jbchem.a122558. [DOI] [PubMed] [Google Scholar]
- Karey K. P., Sirbasku D. A. Glutaraldehyde fixation increases retention of low molecular weight proteins (growth factors) transferred to nylon membranes for western blot analysis. Anal Biochem. 1989 May 1;178(2):255–259. doi: 10.1016/0003-2697(89)90634-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Mervis R. J., Ahmad N., Lillehoj E. P., Raum M. G., Salazar F. H., Chan H. W., Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988 Nov;62(11):3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A., Rosenblatt J. D., Chen I. S., DiVincenzo J. P., Bever R., Poiesz B. J., Abraham G. N. Detection and cloning of new HTLV-related endogenous sequences in man. Nucleic Acids Res. 1989 Sep 12;17(17):6841–6854. doi: 10.1093/nar/17.17.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L. H., Yerly S., Adami N., Bachmann P., Bütler-Brunner E., Burckhardt J., Kawashima E. Human immunodeficiency virus DNA amplification and serology in blood donors. Blood. 1990 Aug 1;76(3):641–645. [PubMed] [Google Scholar]
- Sarin P. S., Sun D. K., Thornton A. H., Naylor P. H., Goldstein A. L. Neutralization of HTLV-III/LAV replication by antiserum to thymosin alpha 1. Science. 1986 May 30;232(4754):1135–1137. doi: 10.1126/science.3010464. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Sarngadharan M. G., Gallo R. C. Antigens on HTLV-infected cells recognized by leukemia and AIDS sera are related to HTLV viral glycoprotein. Science. 1984 May 11;224(4649):607–610. doi: 10.1126/science.6324349. [DOI] [PubMed] [Google Scholar]
- Talal N., Garry R. F., Schur P. H., Alexander S., Dauphinée M. J., Livas I. H., Ballester A., Takei M., Dang H. A conserved idiotype and antibodies to retroviral proteins in systemic lupus erythematosus. J Clin Invest. 1990 Jun;85(6):1866–1871. doi: 10.1172/JCI114647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribe D. E., Reed D. L., Lindell P., Kenealy W. R., Ferguson B. Q., Cybulski R., Winslow D., Waselefsky D. M., Petteway S. R., Jr Antibodies reactive with human immunodeficiency virus gag-coded antigens (gag reactive only) are a major cause of enzyme-linked immunosorbent assay reactivity in a blood donor population. J Clin Microbiol. 1988 Apr;26(4):641–647. doi: 10.1128/jcm.26.4.641-647.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A. D., Shaw J., Paetkau V., Bleackley R. C., Magnuson N. S., Reeves R., Magnuson J. A. Cloning of cDNA for the bovine IL-2 receptor (bovine Tac antigen). Immunology. 1988 Apr;63(4):603–610. [PMC free article] [PubMed] [Google Scholar]
- Wittwer C. T., Smith A. M., Ash K. O., DeWitt C. W. False-positive antibody tests for human immunodeficiency virus in transplant patients with antilymphocyte antibodies. Transplantation. 1987 Dec;44(6):843–844. [PubMed] [Google Scholar]