Abstract
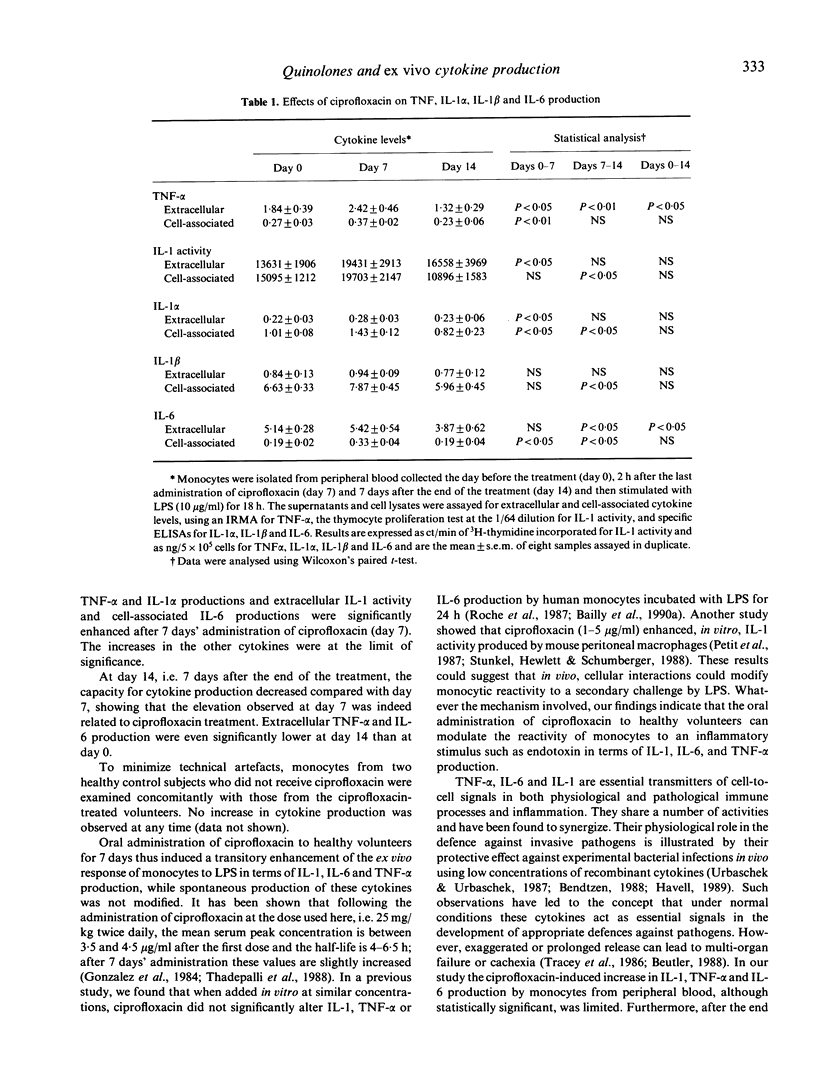
Because in vitro treatment with quinolones, at pharmacological concentrations, modifies lipopolysaccharide (LPS) induced production of cytokines by monocytes, we studied the effect of orally administered ciprofloxacin (25 mg/kg) on the capacity of peripheral blood monocytes of healthy volunteers to produce tumour necrosis factor-alpha (TNF-alpha), IL-1 activity, IL-1 alpha, IL-1 beta and IL-6 ex vivo in response to endotoxin stimulation. After 7 days of ciprofloxacin, the extracellular and cellular production of TNF-alpha, the cellular production of IL-1 activity, the extracellular and cellular production of IL-1 alpha, and the cellular production of IL-6 increased significantly. Seven days after the end of the treatment, values returned to basal levels or even lower. To our knowledge, this is the first demonstration that ciprofloxacin can modulate in vivo the capacity of human monocytes to react to an inflammatory stimulus such as endotoxin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly S., Fay M., Roche Y., Gougerot-Pocidalo M. A. Effects of quinolones on tumor necrosis factor production by human monocytes. Int J Immunopharmacol. 1990;12(1):31–36. doi: 10.1016/0192-0561(90)90065-u. [DOI] [PubMed] [Google Scholar]
- Bailly S., Mahe Y., Ferrua B., Fay M., Tursz T., Wakasugi H., Gougerot-Pocidalo M. A. Quinolone-induced differential modification of IL-1 alpha and IL-1 beta production by LPS-stimulated human monocytes. Cell Immunol. 1990 Jun;128(1):277–288. doi: 10.1016/0008-8749(90)90025-m. [DOI] [PubMed] [Google Scholar]
- Bendtzen K. Interleukin 1, interleukin 6 and tumor necrosis factor in infection, inflammation and immunity. Immunol Lett. 1988 Nov;19(3):183–191. doi: 10.1016/0165-2478(88)90141-1. [DOI] [PubMed] [Google Scholar]
- Beutler B. The presence of cachectin/tumor necrosis factor in human disease states. Am J Med. 1988 Sep;85(3):287–288. doi: 10.1016/0002-9343(88)90575-x. [DOI] [PubMed] [Google Scholar]
- Dalhoff A., Weidner W. Diffusion of ciprofloxacin into prostatic fluid. Eur J Clin Microbiol. 1984 Aug;3(4):360–362. doi: 10.1007/BF01977495. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Ferrua B., Becker P., Schaffar L., Shaw A., Fehlmann M. Detection of human IL-1 alpha and IL-1 beta at the subpicomolar level by colorimetric sandwich enzyme immunoassay. J Immunol Methods. 1988 Nov 10;114(1-2):41–48. doi: 10.1016/0022-1759(88)90151-2. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Bergh A. K., Brandt M., Hansson G. Quinolones affect thymidine incorporation into the DNA of human lymphocytes. Antimicrob Agents Chemother. 1986 Mar;29(3):506–508. doi: 10.1128/aac.29.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. A., Uribe F., Moisen S. D., Fuster A. P., Selen A., Welling P. G., Painter B. Multiple-dose pharmacokinetics and safety of ciprofloxacin in normal volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):741–744. doi: 10.1128/aac.26.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Larrick J. W., Kunkel S. L. The role of tumor necrosis factor and interleukin 1 in the immunoinflammatory response. Pharm Res. 1988 Mar;5(3):129–139. doi: 10.1023/a:1015904721223. [DOI] [PubMed] [Google Scholar]
- Petit J. C., Daguet G. L., Richard G., Burghoffer B. Influence of ciprofloxacin and piperacillin on interleukin-1 production by murine macrophages. J Antimicrob Chemother. 1987 Oct;20(4):615–617. doi: 10.1093/jac/20.4.615. [DOI] [PubMed] [Google Scholar]
- Roche Y., Fay M., Gougerot-Pocidalo M. A. Effects of quinolones on interleukin 1 production in vitro by human monocytes. Immunopharmacology. 1987 Apr;13(2):99–109. doi: 10.1016/0162-3109(87)90046-4. [DOI] [PubMed] [Google Scholar]
- Roche Y., Fay M., Gougerot-Pocidalo M. A. Enhancement of interleukin 2 production by quinolone-treated human mononuclear leukocytes. Int J Immunopharmacol. 1988;10(2):161–167. doi: 10.1016/0192-0561(88)90091-4. [DOI] [PubMed] [Google Scholar]
- Scala G., Oppenheim J. J. Antigen presentation by human monocytes: evidence for stimulant processing and requirement for interleukin 1. J Immunol. 1983 Sep;131(3):1160–1166. [PubMed] [Google Scholar]
- Thadepalli H., Bansal M. B., Rao B., See R., Chuah S. K., Marshall R., Dhawan V. K. Ciprofloxacin: in vitro, experimental, and clinical evaluation. Rev Infect Dis. 1988 May-Jun;10(3):505–515. doi: 10.1093/clinids/10.3.505. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Urbaschek R., Urbaschek B. Tumor necrosis factor and interleukin 1 as mediators of endotoxin-induced beneficial effects. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S607–S615. doi: 10.1093/clinids/9.supplement_5.s607. [DOI] [PubMed] [Google Scholar]


