Abstract
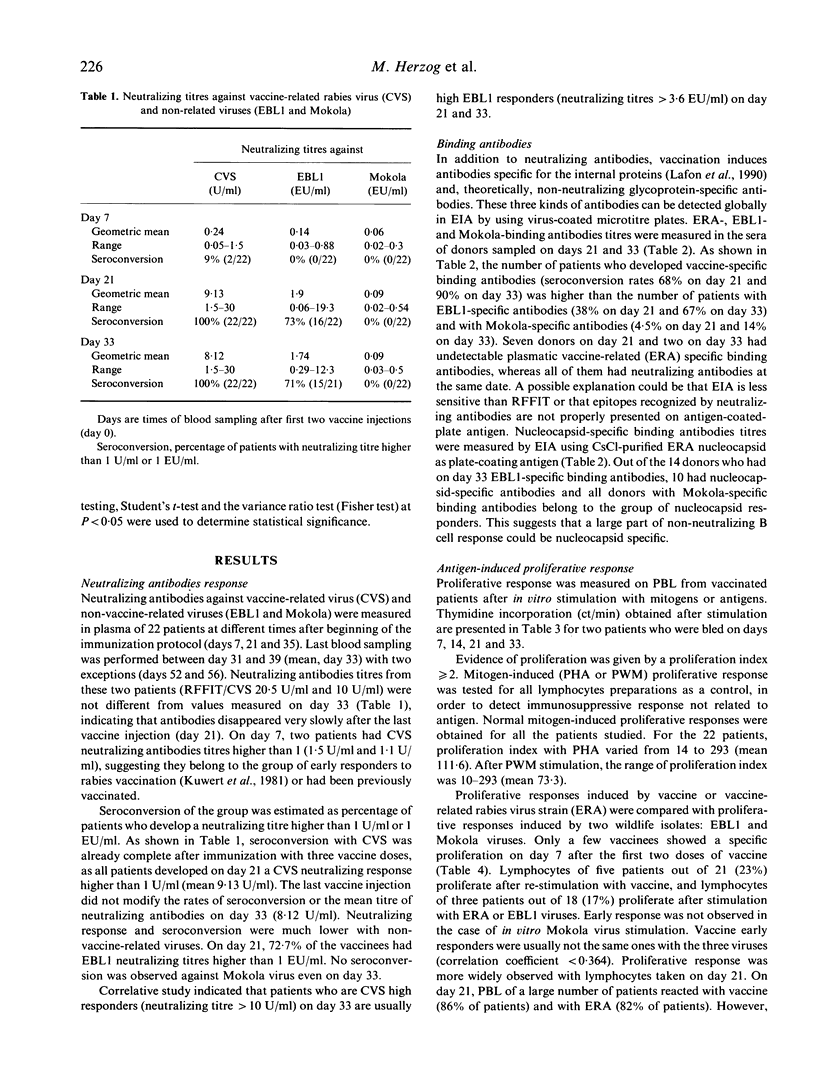
T and B cell human responses to European bat lyssavirus (EBL1) induced by post-exposure rabies vaccination (PM virus vaccine) were evaluated by measuring plasmatic titres of EBL1-specific neutralizing antibodies; specific EBL1-binding antibodies; and proliferation indices of peripheral blood lymphocytes stimulated in vitro with EBL1. These parameters for vaccination efficacy were compared with those obtained with vaccine-related viruses (CVS and ERA) and with a non-vaccine-related virus. Mokola virus, the last implicated in vaccination failures. Twenty-two patients exposed to rabies risk who received a reduced rabies post-exposure vaccination were involved in the study. On day 21, vaccine induced CVS-specific neutralizing antibodies in all patients; but EBL1-specific neutralizing antibodies were induced in only 73% of patients. No vaccine had Mokola-specific neutralizing antibodies. Patients having EBL1-specific neutralizing antibodies were usually those in whom vaccination induced high titres of CVS-specific neutralizing antibodies. On day 21, peripheral blood lymphocytes of 86% of patients could be restimulated in vitro with vaccine, 43% with EBL1 and 45% with Mokola. Patients exhibiting a high vaccine-specific proliferation response more likely developed an EBL1- or a Mokola-specific proliferative response. No correlation was found between T and B cell responses. Rabies vaccination induced neither T nor B cell EBL1-specific responses in 22% of patients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atanasiu P., Savy V., Perrin P. Epreuve immunoenzymatique pour la détection rapide des anticorps antirabiques. Ann Microbiol (Paris) 1977 May-Jun;128A(4):489–498. [PubMed] [Google Scholar]
- Bunschoten H., Klapmuts R. J., Claassen I. J., Reyneveld S. D., Osterhaus A. D., Uytdehaag F. G. Rabies virus-specific human T cell clones provide help for an in vitro antibody response against neutralizing antibody-inducing determinants of the viral glycoprotein. J Gen Virol. 1989 Jun;70(Pt 6):1513–1521. doi: 10.1099/0022-1317-70-6-1513. [DOI] [PubMed] [Google Scholar]
- Celis E., Ou D., Dietzschold B., Koprowski H. Recognition of rabies and rabies-related viruses by T cells derived from human vaccine recipients. J Virol. 1988 Sep;62(9):3128–3134. doi: 10.1128/jvi.62.9.3128-3134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton K. M., Casey G. A. Experimental rabies in skunks: immunofluorescence light and electron microscopic studies. Lab Invest. 1979 Jul;41(1):36–44. [PubMed] [Google Scholar]
- Dietzschold B., Rupprecht C. E., Tollis M., Lafon M., Mattei J., Wiktor T. J., Koprowski H. Antigenic diversity of the glycoprotein and nucleocapsid proteins of rabies and rabies-related viruses: implications for epidemiology and control of rabies. Rev Infect Dis. 1988 Nov-Dec;10 (Suppl 4):S785–S798. doi: 10.1093/clinids/10.supplement_4.s785. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Tollis M., Rupprecht C. E., Celis E., Koprowski H. Antigenic variation in rabies and rabies-related viruses: cross-protection independent of glycoprotein-mediated virus-neutralizing antibody. J Infect Dis. 1987 Nov;156(5):815–822. doi: 10.1093/infdis/156.5.815. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Wang H. H., Rupprecht C. E., Celis E., Tollis M., Ertl H., Heber-Katz E., Koprowski H. Induction of protective immunity against rabies by immunization with rabies virus ribonucleoprotein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9165–9169. doi: 10.1073/pnas.84.24.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foggin C. M. Atypical rabies virus in cats and a dog in Zimbabwe. Vet Rec. 1982 Apr 3;110(14):338–338. doi: 10.1136/vr.110.14.338. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lafon M., Bourhy H., Sureau P. Immunity against the European bat rabies (Duvenhage) virus induced by rabies vaccines: an experimental study in mice. Vaccine. 1988 Aug;6(4):362–368. doi: 10.1016/0264-410x(88)90184-3. [DOI] [PubMed] [Google Scholar]
- Lafon M., Edelman L., Bouvet J. P., Lafage M., Montchâtre E. Human monoclonal antibodies specific for the rabies virus glycoprotein and N protein. J Gen Virol. 1990 Aug;71(Pt 8):1689–1696. doi: 10.1099/0022-1317-71-8-1689. [DOI] [PubMed] [Google Scholar]
- Lafon M., Herzog M., Sureau P. Human rabies vaccines induce neutralising antibodies against the European bat rabies virus (Duvenhage) Lancet. 1986 Aug 30;2(8505):515–515. doi: 10.1016/s0140-6736(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Lumio J., Hillbom M., Roine R., Ketonen L., Haltia M., Valle M., Neuvonen E., Lähdevirta J. Human rabies of bat origin in Europe. Lancet. 1986 Feb 15;1(8477):378–378. doi: 10.1016/s0140-6736(86)92336-6. [DOI] [PubMed] [Google Scholar]
- Mifune K., Takeuchi E., Napiorkowski P. A., Yamada A., Sakamoto K. Essential Role of T cells in the postexposure prophylaxis of rabies in mice. Microbiol Immunol. 1981;25(9):895–904. doi: 10.1111/j.1348-0421.1981.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Bauer S. P., Harrison A. K., Winn W. C., Jr Comparative pathogenesis of rabies and rabies-like viruses. Viral infection and transit from inoculation site to the central nervous system. Lab Invest. 1973 Mar;28(3):361–376. [PubMed] [Google Scholar]
- Nicholson K. G., Cole P. J., Turner G. S., Harrison P. Immune responses of humans to a human diploid cell strain of rabies virus vaccine: lymphocyte transformation, production of virus-neutralizing antibody, and induction of interferon. J Infect Dis. 1979 Aug;140(2):176–182. doi: 10.1093/infdis/140.2.176. [DOI] [PubMed] [Google Scholar]
- Ratanavongsiri J., Sriwanthana B., Ubol S., Phanuphak P. Cell-mediated immune response following intracutaneous immunisation with human diploid cell rabies vaccine. Asian Pac J Allergy Immunol. 1985 Dec;3(2):187–190. [PubMed] [Google Scholar]
- Sato M., Maeda N., Yoshida H., Urade M., Saito S. Plaque formation of herpes virus hominis type 2 and rubella virus in variants isolated from the colonies of BHK21/WI-2 cells formed in soft agar. Arch Virol. 1977;53(3):269–273. doi: 10.1007/BF01314672. [DOI] [PubMed] [Google Scholar]
- Schneider L. G. Antigenic variants of rabies virus. Comp Immunol Microbiol Infect Dis. 1982;5(1-3):101–107. doi: 10.1016/0147-9571(82)90021-2. [DOI] [PubMed] [Google Scholar]
- Schumacher C. L., Dietzschold B., Ertl H. C., Niu H. S., Rupprecht C. E., Koprowski H. Use of mouse anti-rabies monoclonal antibodies in postexposure treatment of rabies. J Clin Invest. 1989 Sep;84(3):971–975. doi: 10.1172/JCI114260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimov M. A., Tatarov A. G., Botvinkin A. D., Klueva E. V., Kulikova L. G., Khismatullina N. A. Rabies-related Yuli virus; identification with a panel of monoclonal antibodies. Acta Virol. 1989 Dec;33(6):542–546. [PubMed] [Google Scholar]
- Smith J. S. Mouse model for abortive rabies infection of the central nervous system. Infect Immun. 1981 Jan;31(1):297–308. doi: 10.1128/iai.31.1.297-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Yager P. A., Baer G. M. A rapid tissue culture test for determining rabies neutralizing antibody. Monogr Ser World Health Organ. 1973;(23):354–357. [PubMed] [Google Scholar]
- Turner G. S. Thymus dependence of rabies vaccine. J Gen Virol. 1976 Dec;33(3):535–538. doi: 10.1099/0022-1317-33-3-535. [DOI] [PubMed] [Google Scholar]
- Vodopija I., Sureau P., Smerdel S., Lafon M., Baklaić Z., Ljubicić M., Svjetlicić M. Interaction of rabies vaccine with human rabies immunoglobulin and reliability of a 2-1-1 schedule application for postexposure treatment. Vaccine. 1988 Jun;6(3):283–286. doi: 10.1016/0264-410x(88)90225-3. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Dietzschold B., Leamnson R. N., Koprowski H. Induction and biological properties of defective interfering particles of rabies virus. J Virol. 1977 Feb;21(2):626–635. doi: 10.1128/jvi.21.2.626-635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., György E., Schlumberger D., Sokol F., Koprowski H. Antigenic properties of rabies virus components. J Immunol. 1973 Jan;110(1):269–276. [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Foggin C. M., Koprowski H. Antigenic analysis of rabies and Mokola virus from Zimbabwe using monoclonal antibodies. Dev Biol Stand. 1984;57:199–211. [PubMed] [Google Scholar]
- Zalan E., Wilson C., Pukitis D. A microtest for the quantitation of rabies virus neutralizing antibodies. J Biol Stand. 1979 Jul;7(3):213–220. doi: 10.1016/s0092-1157(79)80024-4. [DOI] [PubMed] [Google Scholar]


