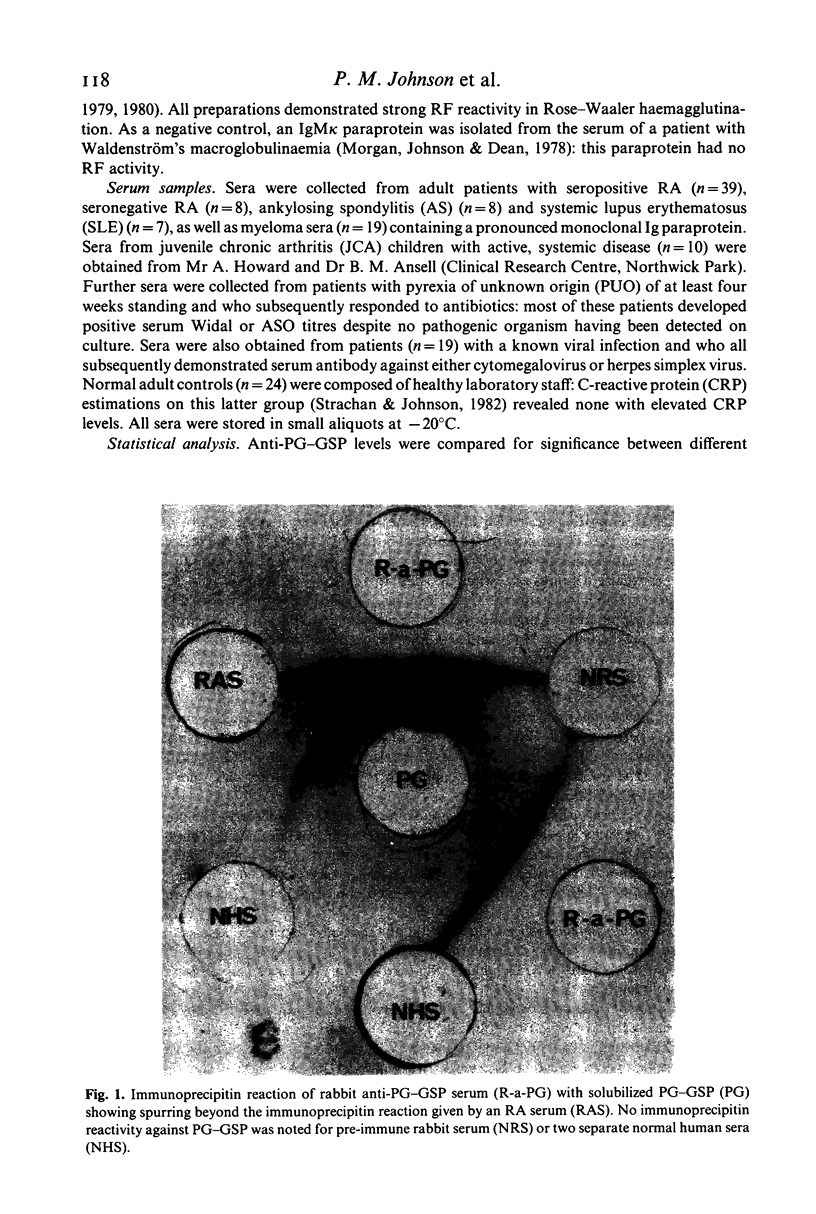
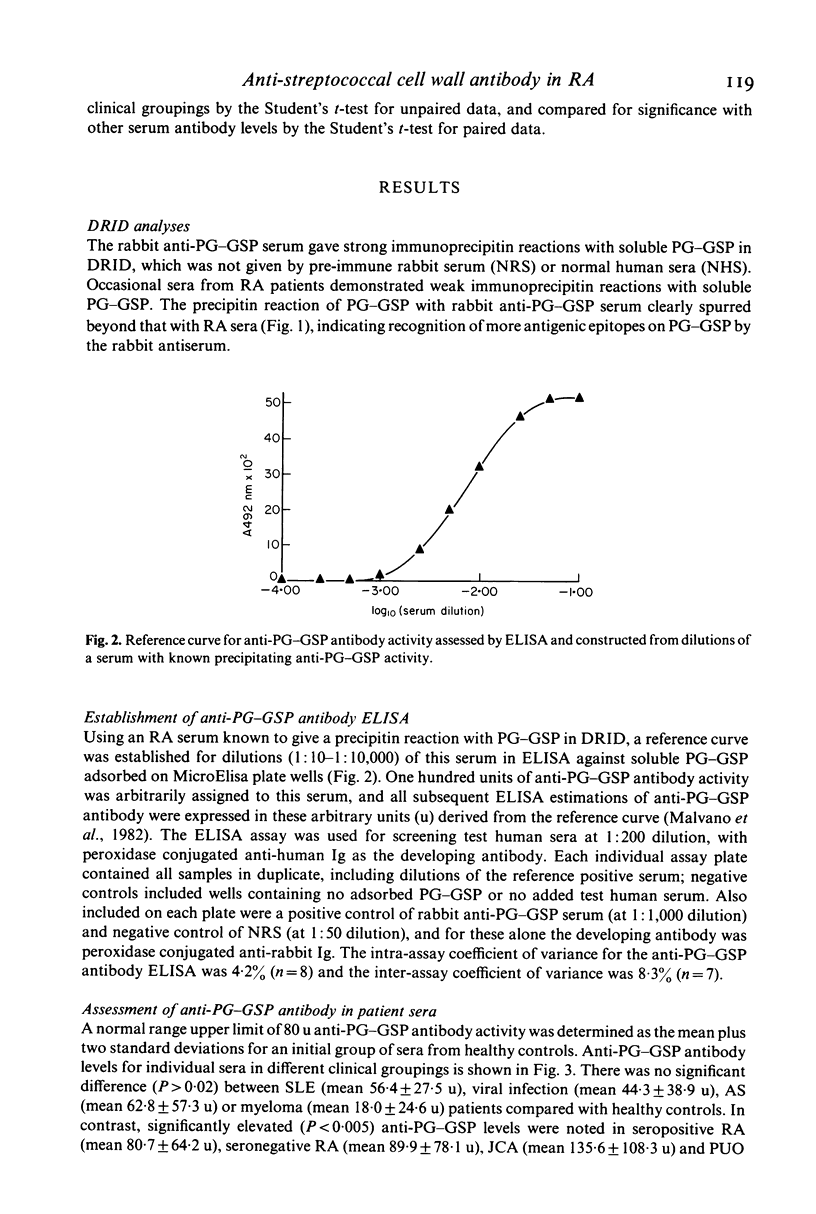
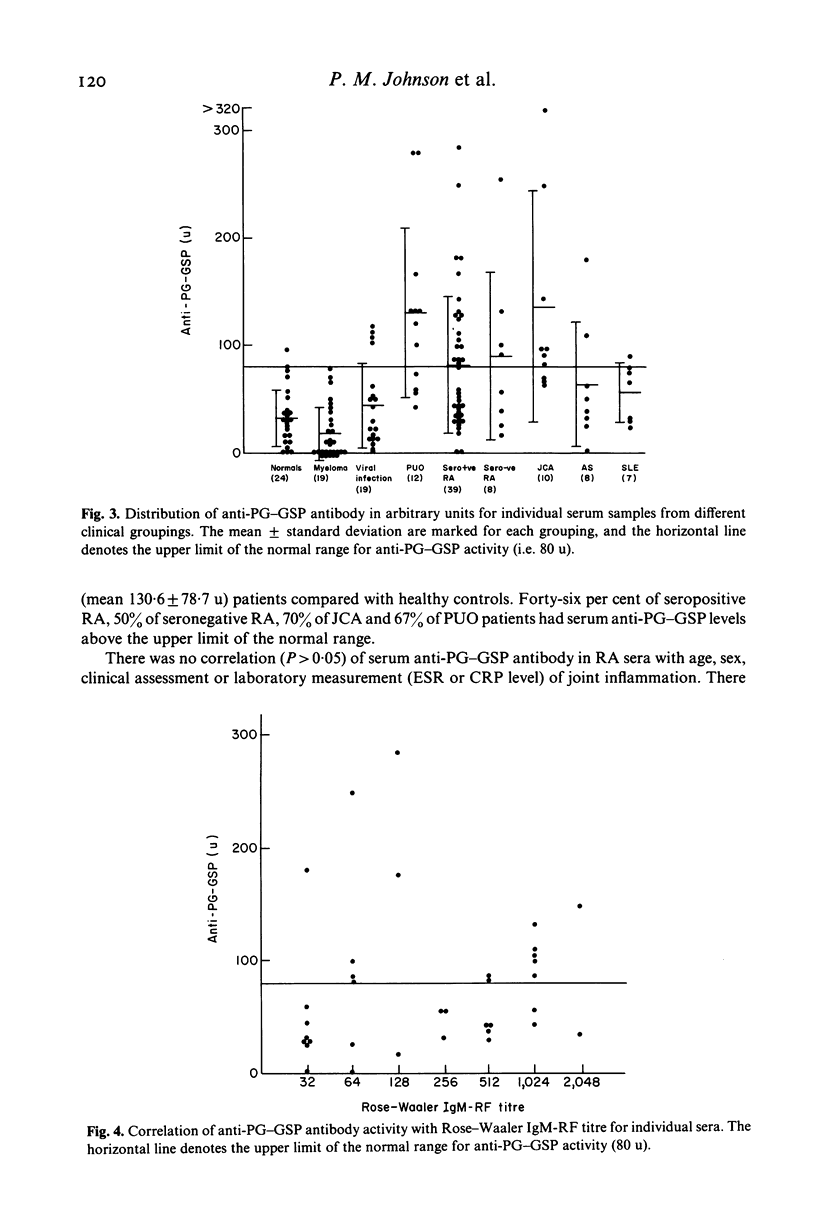
Abstract
An ELISA has been developed for serum antibodies to streptococcal cell wall peptidoglycan-polysaccharide polymers (PG-GSP). A significantly increased prevalence of serum anti-PG-GSP antibody was found in juvenile chronic arthritis and both seropositive and seronegative rheumatoid arthritis (RA), compared with ankylosing spondylitis, systemic lupus erythematosus, myeloma and healthy controls. Anti-PG-GSP antibody was always of the IgG class and there was no correlation of anti-PG-GSP levels with C reactive protein, rheumatoid factor (RF) or anti-streptolysin O titres. There was no direct cross-reaction of RF with PG-GSP, nor did the presence of IgM-RF significantly interfere with the assay. Examination of paired serum and synovial fluid samples offered no evidence for local production of anti-PG-GSP antibody in synovial tissue. These data are compatible with an increased systemic immunization by bacterial fragments in RA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. C. The infectious etiology of rheumatoid arthritis. New considerations. Arthritis Rheum. 1978 Jun;21(5):531–538. doi: 10.1002/art.1780210507. [DOI] [PubMed] [Google Scholar]
- Bokisch V. A., Chiao J. W., Bernstein D., Krause R. M. Homogenous rabbit 7S anti-IgG with antibody specificity for peptidoglycan. J Exp Med. 1973 Nov 1;138(5):1184–1193. doi: 10.1084/jem.138.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Preferential induction of autoantibody secretion in polyclonal activation by peptidoglycan and lipopolysaccharide. I. In vitro studies. J Immunol. 1982 Mar;128(3):1018–1025. [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J. J., Hunter N., Schwab J. H. Antibody response to streptococcal cell wall antigens associated with experimental arthritis in rats. Clin Exp Immunol. 1980 Dec;42(3):450–457. [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymer B., Schleifer K. H., Read S., Zabriskie J. B., Krause R. M. Detection of antibodies to bacterial cell wall peptidoglycan in human sera. J Immunol. 1976 Jul;117(1):23–26. [PubMed] [Google Scholar]
- Hobbs R. N., Lea D. J., Phua K. K., Johnson P. M. Binding of isolated rheumatoid factors to histone proteins and basic polycations. Ann Rheum Dis. 1983 Aug;42(4):435–438. doi: 10.1136/ard.42.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. M. IgM-rheumatoid factors cross-reactive with IgG and a cell nuclear antigen: apparent 'masking' in original serum. Scand J Immunol. 1979;9(5):461–466. doi: 10.1111/j.1365-3083.1979.tb03068.x. [DOI] [PubMed] [Google Scholar]
- Johnson P. M. IgM-rheumatoid factors cross-reactive with IgG and a cell nuclear antigen: immunopathological implications? Ann Rheum Dis. 1980 Dec;39(6):586–588. doi: 10.1136/ard.39.6.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettering J. D., Schmidt N. J., Lennette E. H. Improved glycine-extracted complement-fixing antigen for human cytomegalovirus. J Clin Microbiol. 1977 Dec;6(6):647–649. doi: 10.1128/jcm.6.6.647-649.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris J. D., Allen J. B., Schwab J. H. In vivo changes in complement induced with peptidoglycan-polysaccharide polymers from streptococcal cell walls. Infect Immun. 1982 Jan;35(1):377–380. doi: 10.1128/iai.35.1.377-380.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvano R., Boniolo A., Dovis M., Zannino M. ELISA for antibody measurement: aspects related to data expression. J Immunol Methods. 1982;48(1):51–60. doi: 10.1016/0022-1759(82)90209-5. [DOI] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R. Modifications of the acyl-D-alanyl-D-alanine terminus affecting complex-formation with vancomycin. Biochem J. 1971 Aug;123(5):789–803. doi: 10.1042/bj1230789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. F., Munoz E., Ghuysen J. M. Peptide cross-links in bacterial cell wall peptidoglycans studied with specific endopeptidases from Streptomyces albus G. Biochemistry. 1966 Aug;5(8):2764–2776. doi: 10.1021/bi00872a037. [DOI] [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E., ZUCKERMAN A. Hemolysis and hemagglutination by normal and immune serums of erythrocytes treated with a nonspecies specific bacterial substance. J Infect Dis. 1956 Mar-Apr;98(2):211–222. doi: 10.1093/infdis/98.2.211. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Krause R. M. The immunochemistry of peptidoglycan. I. The immunodominant site of the peptide subunit and the contribution of each of the amino acids to the binding properties of the peptides. J Biol Chem. 1971 Feb 25;246(4):986–993. [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Cytotoxicity of rat macrophages activated by persistent or biodegradable bacterial cell walls. Infect Immun. 1977 Sep;17(3):599–606. doi: 10.1128/iai.17.3.599-606.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowicz R. J., Schwab J. H. Processing of streptococcal cell walls by rat macrophages and human monocytes in vitro. Infect Immun. 1977 Sep;17(3):591–598. doi: 10.1128/iai.17.3.591-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Tull D. E. The immunological activities of bacterial peptidoglycans. Annu Rev Microbiol. 1980;34:311–340. doi: 10.1146/annurev.mi.34.100180.001523. [DOI] [PubMed] [Google Scholar]
- Strachan A. F., Johnson P. M. Protein SAP (serum amyloid P-component) in Waldenström's macroglobulinaemia, multiple myeloma and rheumatic diseases. J Clin Lab Immunol. 1982 Sep;8(3):153–156. [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]