Abstract
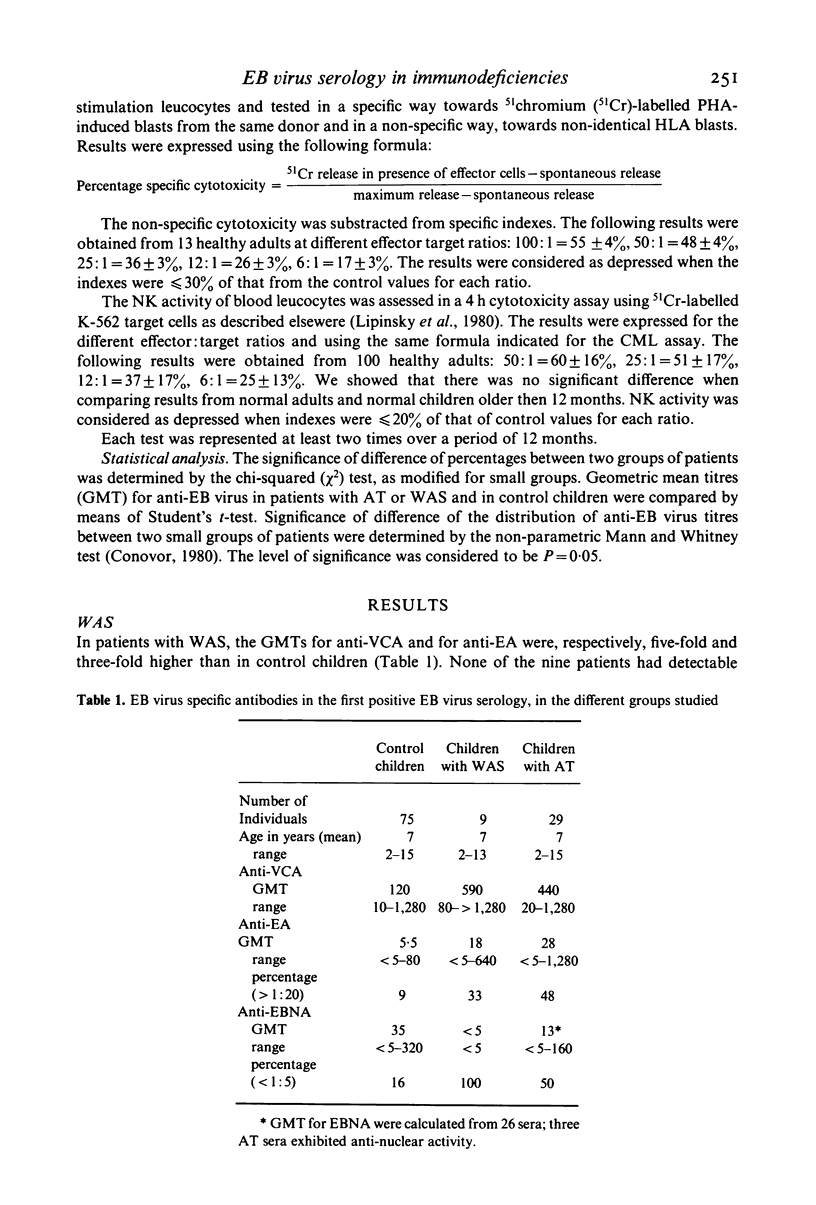
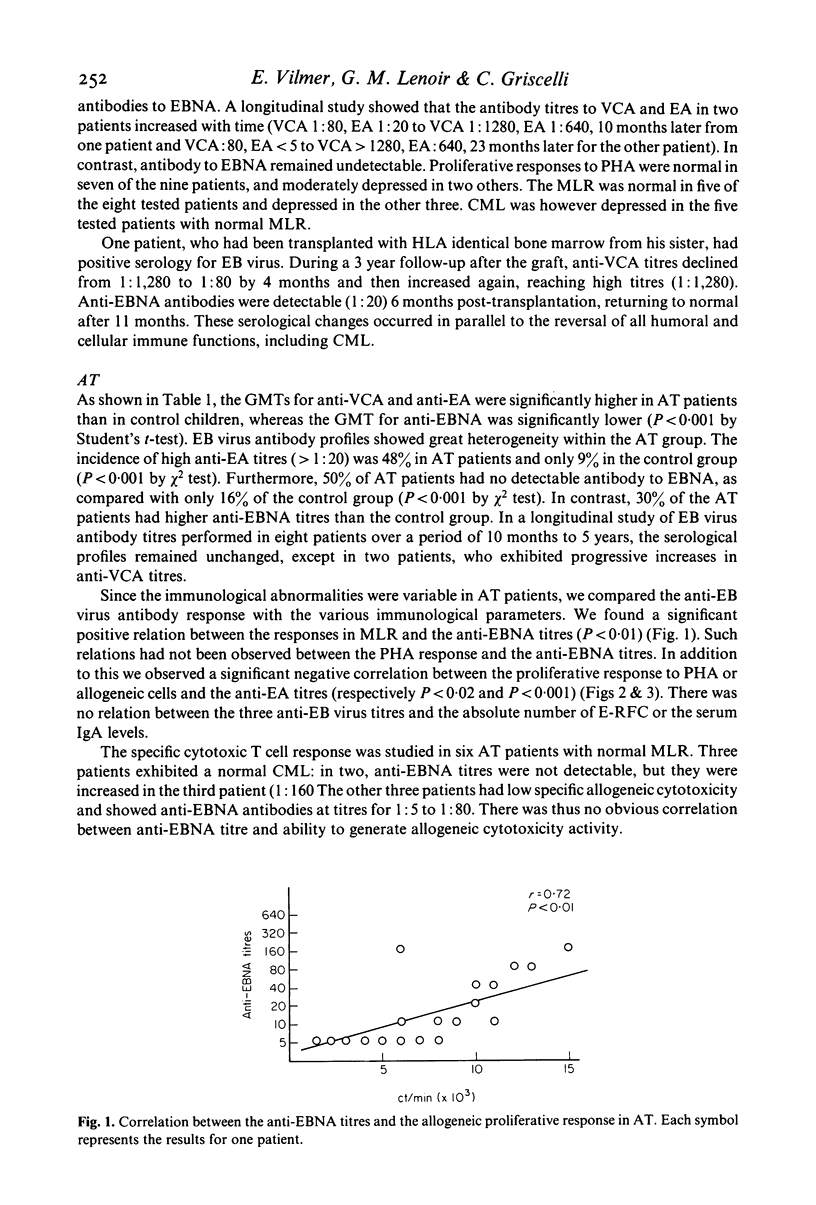
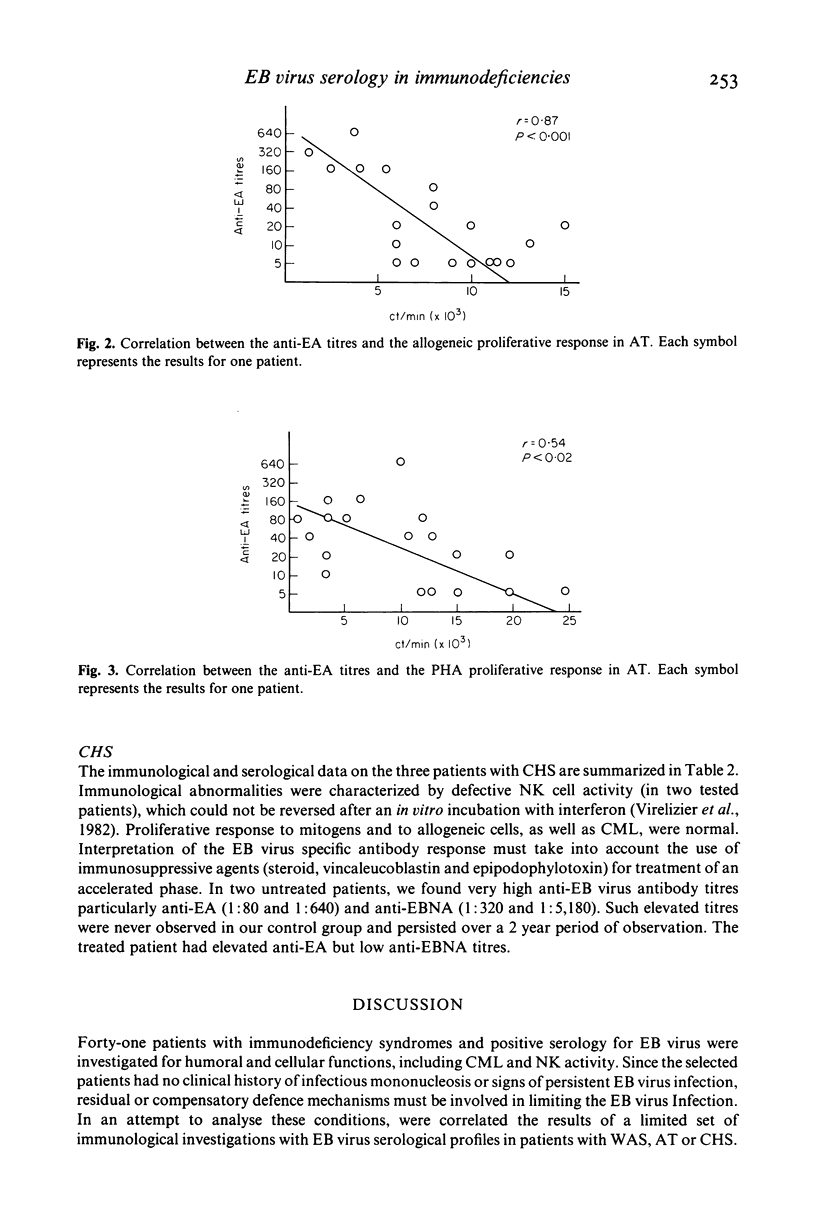
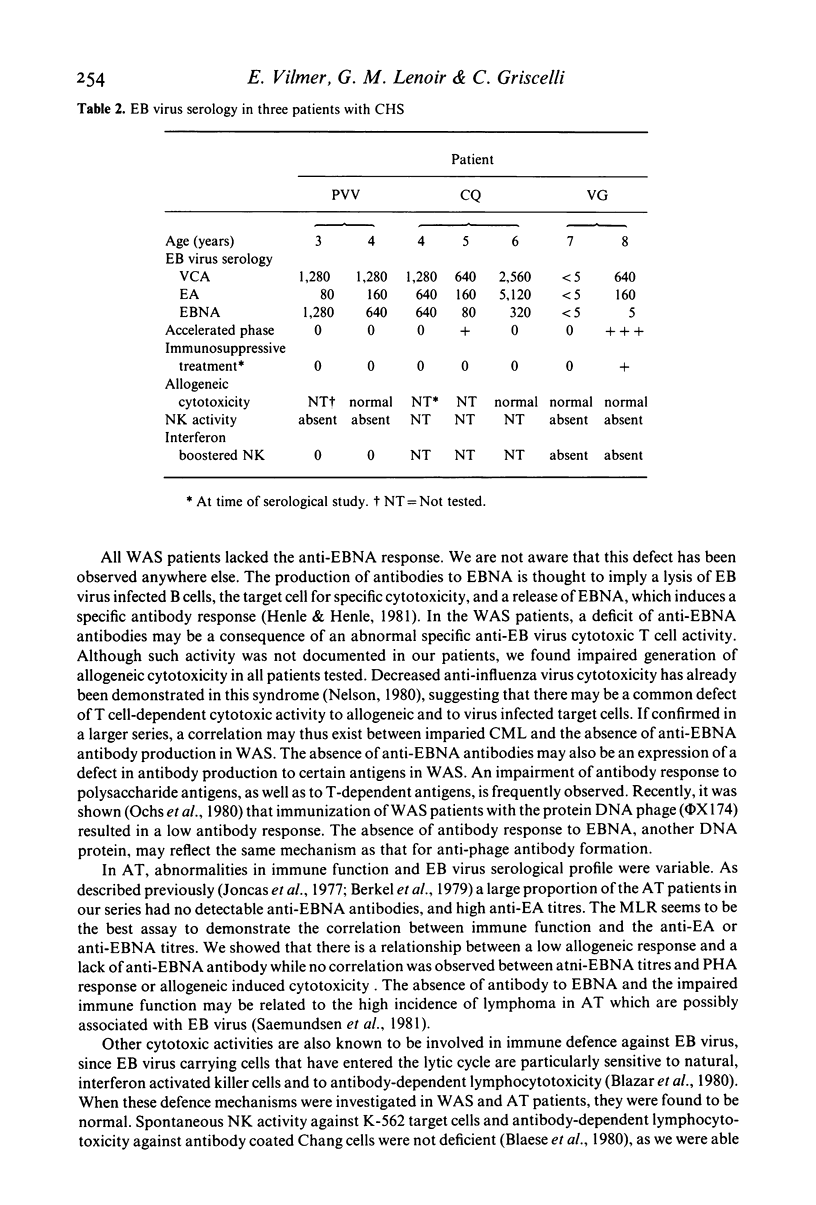
Epstein-Barr (EB) virus serology was correlated with the results of immunological investigations of three inherited immunodeficiency diseases, in an attempt to understand the immune mechanisms controlling EB virus infection. In nine patients with Wiskott-Aldrich syndrome (WAS), the constant lack of anti-EB virus associated nuclear antigen (EBNA) was accompanied by a consistent impairment of allogeneic cytotoxicity. We confirmed a frequent absence of anti-EBNA antibody in ataxia telangiectasia (AT), and we showed a correlation between the level of anti-EBNA response and the mixed leucocyte response (MLR), i.e., an absence of anti-EBNA antibody correlated with a decreased MLR. In two of three untreated patients with Chediak-Higashi syndrome (CHS), high persistent titres of anti-EA antibodies were observed, which were possibly related to a defective natural killer (NK) cell activity. In spite of previous infection with EB virus, none of the 41 patients exhibited clinical signs attributable to the virus, suggesting that residual or compensatory mechanisms must have limited activation of the virus. In patients with AT and WAS these mechanisms may include NK cell activity, which is not depressed in these syndromes, whereas in patients with CHS, they may involve T cell cytotoxicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkel A. I., Henle W., Henle G., Klein G., Ersoy F., Sanal O. Epstein-Barr virus-related antibody patterns in ataxia-telangiectasia. Clin Exp Immunol. 1979 Feb;35(2):196–201. [PMC free article] [PubMed] [Google Scholar]
- Blazar B., Patarroyo M., Klein E., Klein G. Increased sensitivity of human lymphoid lines to natural killer cells after induction of the Epstein-Barr viral cycle by superinfection or sodium butyrate. J Exp Med. 1980 Mar 1;151(3):614–627. doi: 10.1084/jem.151.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durandy A., Wioland M., Sabolovic D., Griscelli C. Electrophoretic characteristics and membrane receptors of lymphocytes in primary immunodeficiency diseases. Clin Immunol Immunopathol. 1975 Sep;4(3):440–448. doi: 10.1016/0090-1229(75)90012-4. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G. E., Horwitz C. A. Epstein-Barr virus specific diagnostic tests in infectious mononucleosis. Hum Pathol. 1974 Sep;5(5):551–565. doi: 10.1016/s0046-8177(74)80006-7. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G. Epstein-Barr virus-specific serology in immunologically compromised individuals. Cancer Res. 1981 Nov;41(11 Pt 1):4222–4225. [PubMed] [Google Scholar]
- Hewetson J. F., Rocchi G., Henle W., Henle G. Neutralizing antibodies to Epstein-Barr virus in healthy populations and patients with infectious mononucleosis. J Infect Dis. 1973 Sep;128(3):283–289. doi: 10.1093/infdis/128.3.283. [DOI] [PubMed] [Google Scholar]
- Joncas J., Lapointe N., Gervais F., Leyritz M. Unusual prevalence of Epstein-Barr virus early antigen (EBV-EA) antibodies in ataxia telangiectasia. J Immunol. 1977 Nov;119(5):1857–1859. [PubMed] [Google Scholar]
- Klein E., Ernberg I., Masucci M. G., Szigeti R., Wu Y. T., Masucci G., Svedmyr E. T-cell response to B-cells and Epstein-Barr virus antigens in infectious mononucleosis. Cancer Res. 1981 Nov;41(11 Pt 1):4210–4215. [PubMed] [Google Scholar]
- Lenoir G., Tovey M. G., Lavoue M. F. Induction of Epstein-Barr virus early antigen by phytohaemagglutinin in the presence of 5-iodo-2'-deoxyuridine: application to EBV serology. J Immunol Methods. 1980;34(1):23–29. doi: 10.1016/0022-1759(80)90220-3. [DOI] [PubMed] [Google Scholar]
- Lipinski M., Virelizier J. L., Tursz T., Griscelli C. Natural killer and killer cell activities in patients with primary immunodeficiencies or defects in immune interferon production. Eur J Immunol. 1980 Apr;10(4):246–249. doi: 10.1002/eji.1830100405. [DOI] [PubMed] [Google Scholar]
- Mawas C., Sasportes M., Charmot D., Christen Y., Dausset J. Cell-mediated lympholysis in vitro. Independence of mixed lymphocyte reactions and T-cell mitogen responses from the in vitro generation of cytotoxic effectors in primary immunodeficiency diseases. Clin Exp Immunol. 1975 Apr;20(1):83–92. [PMC free article] [PubMed] [Google Scholar]
- Ochs H. D., Slichter S. J., Harker L. A., Von Behrens W. E., Clark R. A., Wedgwood R. J. The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood. 1980 Feb;55(2):243–252. [PubMed] [Google Scholar]
- Pearson G. R., Orr T. W. Antibody-dependent lymphocyte cytotoxicity against cells expressing Epstein-Barr virus antigens. J Natl Cancer Inst. 1976 Mar;56(3):485–488. doi: 10.1093/jnci/56.3.485. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Moss D. J., Wallace L. E., Rowe M., Misko I. S., Epstein M. A., Pope J. H. Long-term T-cell-mediated immunity to Epstein-Barr virus. Cancer Res. 1981 Nov;41(11 Pt 1):4216–4221. [PubMed] [Google Scholar]
- Roder J. C., Haliotis T., Klein M., Korec S., Jett J. R., Ortaldo J., Heberman R. B., Katz P., Fauci A. S. A new immunodeficiency disorder in humans involving NK cells. Nature. 1980 Apr 10;284(5756):553–555. doi: 10.1038/284553a0. [DOI] [PubMed] [Google Scholar]
- Saemundsen A. K., Purtilo D. T., Sakamoto K., Sullivan J. L., Synnerholm A. C., Hanto D., Simmons R., Anvret M., Collins R., Klein G. Documentation of Epstein-Barr virus infection in immunodeficient patients with life-threatening lymphoproliferative diseases by Epstein-Barr virus complementary RNA/DNA and viral DNA/DNA hybridization. Cancer Res. 1981 Nov;41(11 Pt 1):4237–4242. [PubMed] [Google Scholar]
- Sullivan J. L., Byron K. S., Brewster F. E., Purtilo D. T. Deficient natural killer cell activity in x-linked lymphoproliferative syndrome. Science. 1980 Oct 31;210(4469):543–545. doi: 10.1126/science.6158759. [DOI] [PubMed] [Google Scholar]
- Svedmyr E., Jondal M. Cytotoxic effector cells specific for B Cell lines transformed by Epstein-Barr virus are present in patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1622–1626. doi: 10.1073/pnas.72.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Lagrue A., Durandy A., Arenzana F., Oury C., Griscelli C., Reinert P. Reversal of natural killer defect in a patient with Chédiak-Higashi syndrome after bone-marrow transplantation. N Engl J Med. 1982 Apr 29;306(17):1055–1056. doi: 10.1056/nejm198204293061718. [DOI] [PubMed] [Google Scholar]


