Abstract
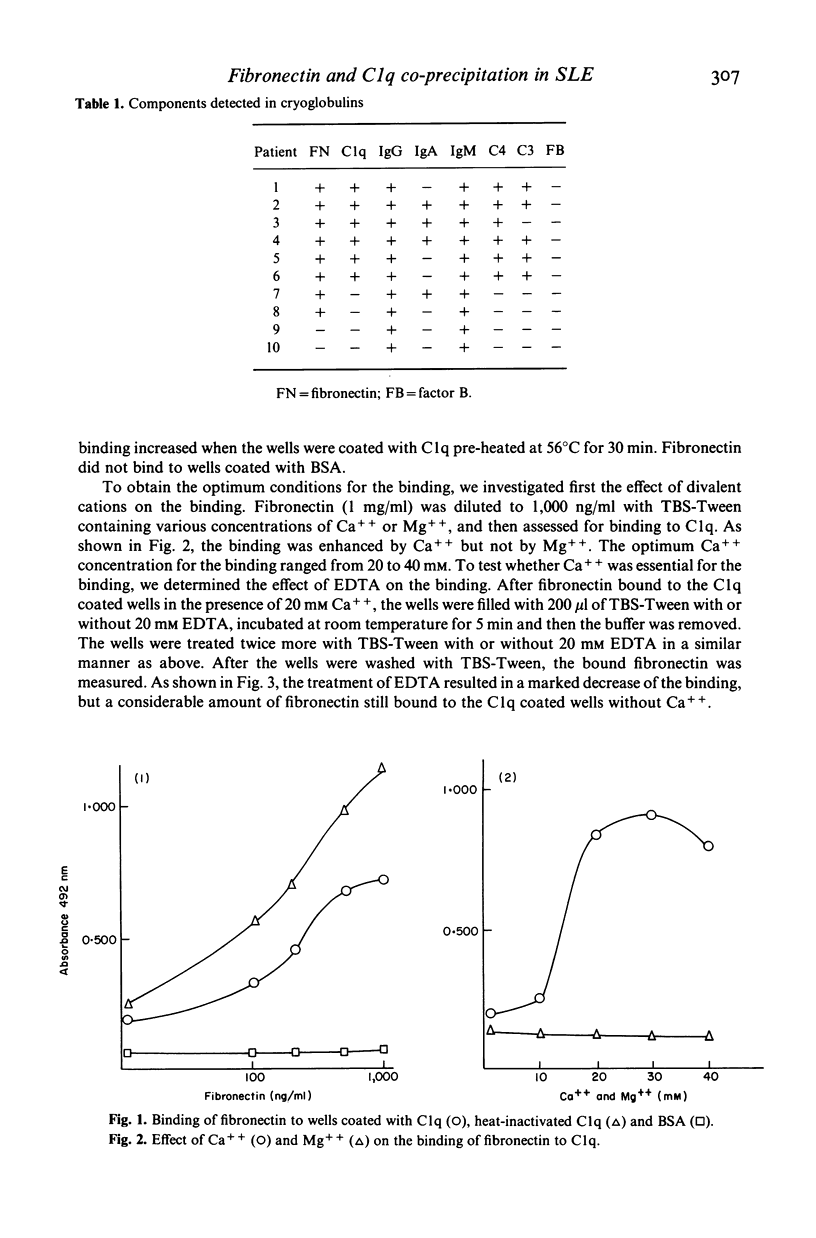
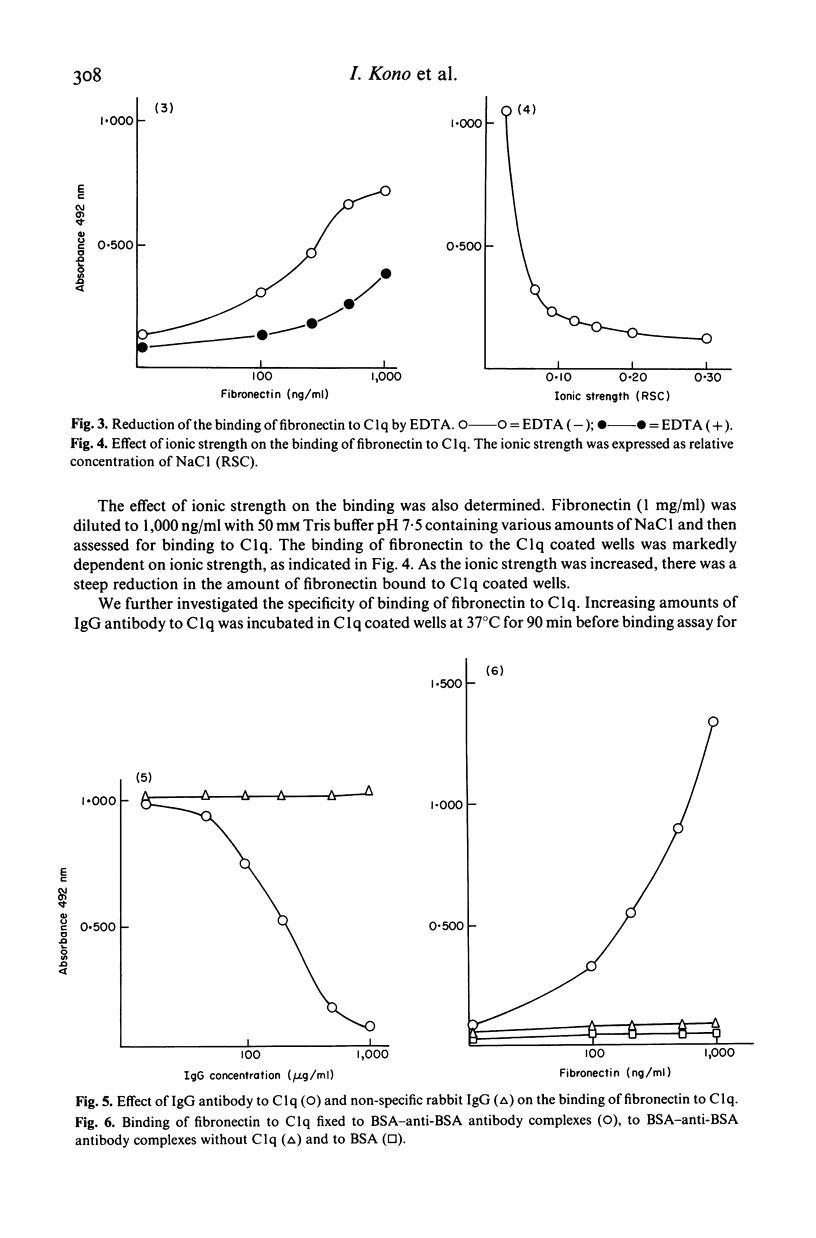
Fibronectin and C1q frequently co-precipitated in cryoglobulins from patients with SLE. As a portion of the C1q molecule is similar to collagen to which fibronectin has a high affinity, we studied whether fibronectin specifically bound to C1q. Fibronectin was found to bind to both native and heat-inactivated C1q. The binding was enhanced by Ca++ and low ionic strength. We have also demonstrated that fibronectin is capable of binding to C1q fixed to immune complexes. The interaction between fibronectin and C1q may play a role in cryoglobulin formation and in clearance of immune complexes by reticuloendothelial system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaulieu A. D., Valet J. P., Strevey J. The influence of fibronectin on cryoprecipitate formation in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 1981 Nov;24(11):1383–1388. doi: 10.1002/art.1780241108. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer L. B., Christian C. L. Studies of cryoproteins in systemic lupus erythematosus. J Clin Invest. 1967 Mar;46(3):400–408. doi: 10.1172/JCI105541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. A. Cryoprecipitogogue from normal serum: mechanism for cryoprecipitation of immune complexes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4562–4565. doi: 10.1073/pnas.78.7.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel E. J., Smolen J. S., Liotta L., Reid K. B. Interaction of fibronectin with C1q and its collagen-like fragment (CLF). FEBS Lett. 1981 Jun 29;129(1):188–192. doi: 10.1016/0014-5793(81)80787-9. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Sorvillo J., Gigli I. The interaction of human plasma fibronectin with a subunit of the first component of complement, C1q. J Immunol. 1982 May;128(5):2036–2039. [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. The biochemistry of complement. Nature. 1978 Oct 26;275(5682):699–704. doi: 10.1038/275699a0. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Jaffe E. Plasma fibronectin (opsonic glycoprotein): its synthesis by vascular endothelial cells and role in cardiopulmonary integrity after trauma as related to reticuloendothelial function. Am J Med. 1980 Apr;68(4):577–594. doi: 10.1016/0002-9343(80)90310-1. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Lesavre P. H., Cooper N. R. Purification and radiolabeling of human C1q. J Immunol. 1981 Aug;127(2):648–653. [PubMed] [Google Scholar]
- Wilson M. R., Arroyave C. M., Miles L., Tan E. M. Immune reactants in cryoproteins. Relationship to complement activation. Ann Rheum Dis. 1977 Dec;36(6):540–548. doi: 10.1136/ard.36.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield J. B., Koffler D., Kunkel H. G. Specific concentration of polynucleotide immune complexes in the cryoprecipitates of patients with systemic lupus erythematosus. J Clin Invest. 1975 Sep;56(3):563–570. doi: 10.1172/JCI108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G., Rucker M., Davis J. W., Entwistle R., Anderson B. Interaction of plasma fibronectin with selected cryoglobulins. Clin Exp Immunol. 1980 May;40(2):358–364. [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Schlesinger D. H., Kennedy D. W., Pastan I. Characterization of a major fibroblast cell surface glycoprotein. Biochemistry. 1977 Dec 13;16(25):5552–5559. doi: 10.1021/bi00644a025. [DOI] [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Active disassembly of the first complement component, C-1, by C-1 inactivator. J Immunol. 1979 Aug;123(2):788–792. [PubMed] [Google Scholar]


