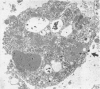
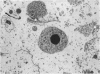
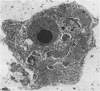
Abstract
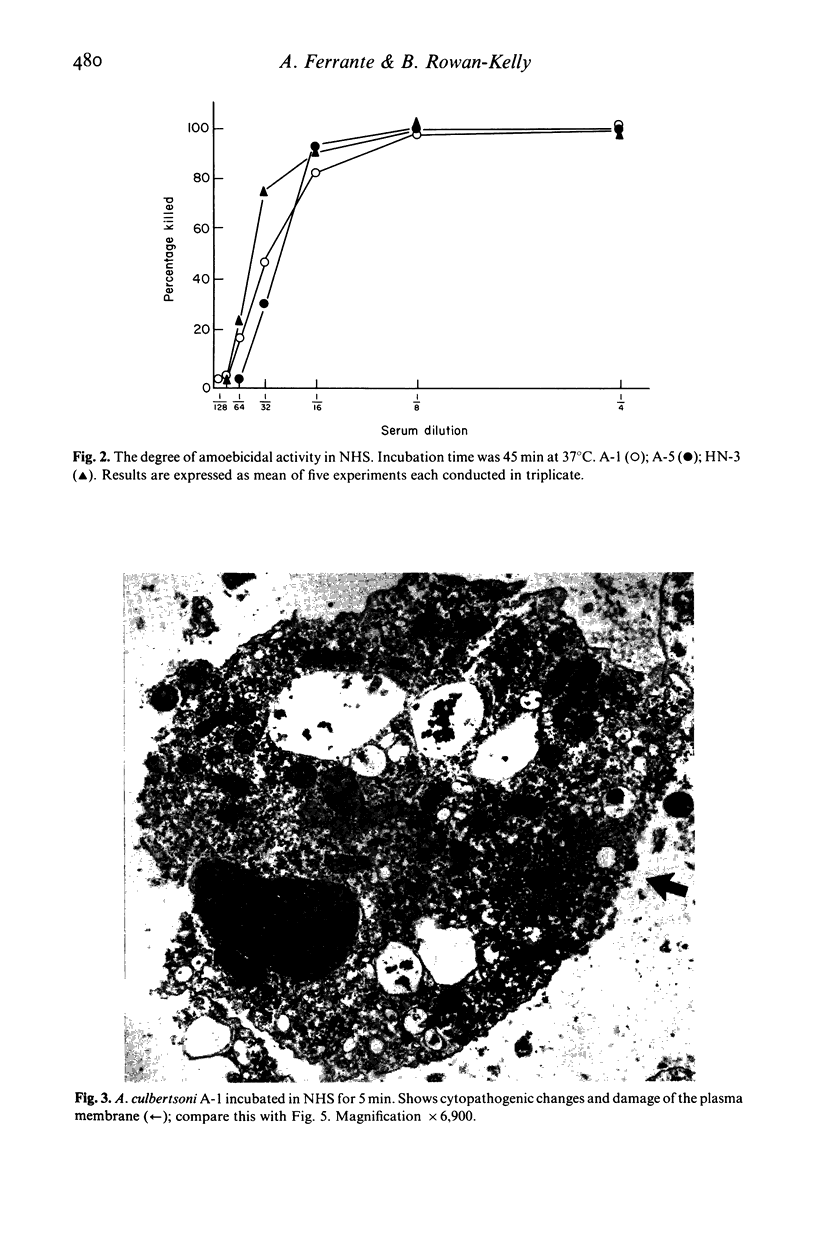
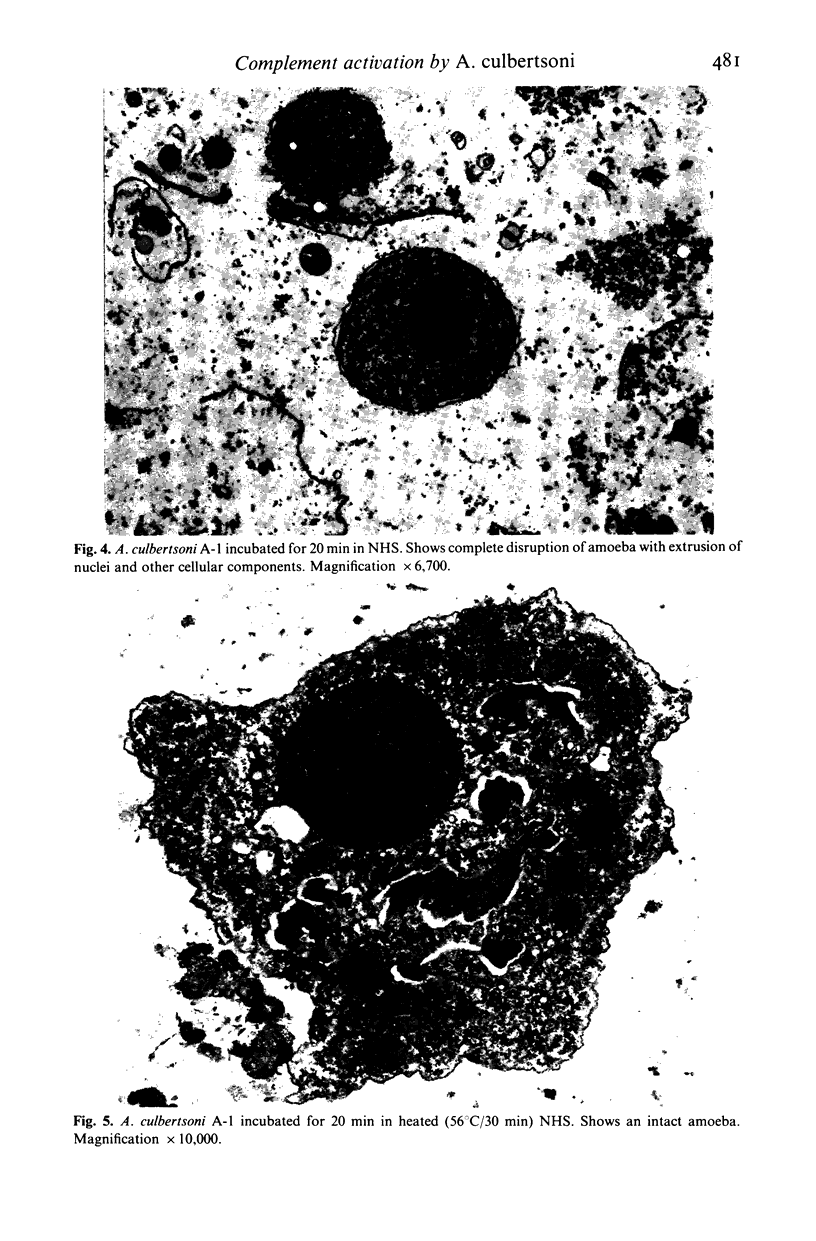
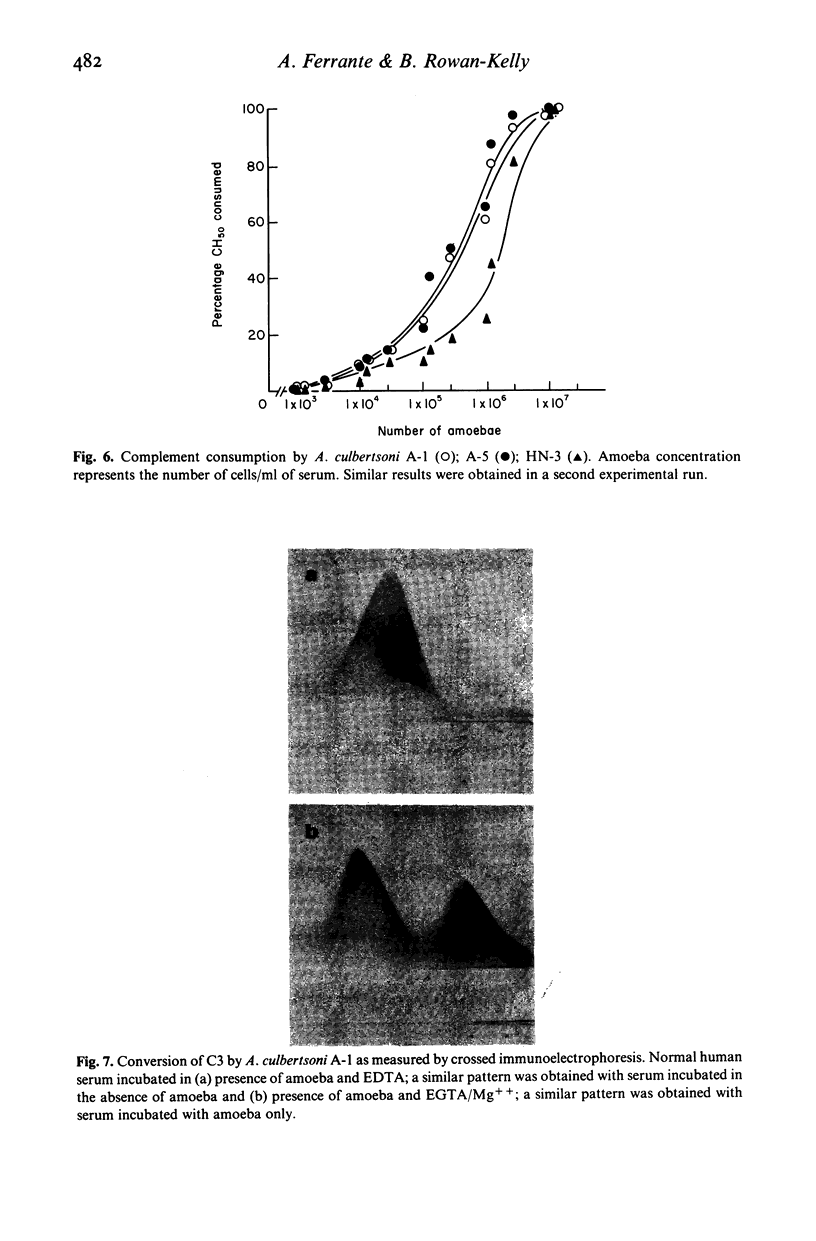
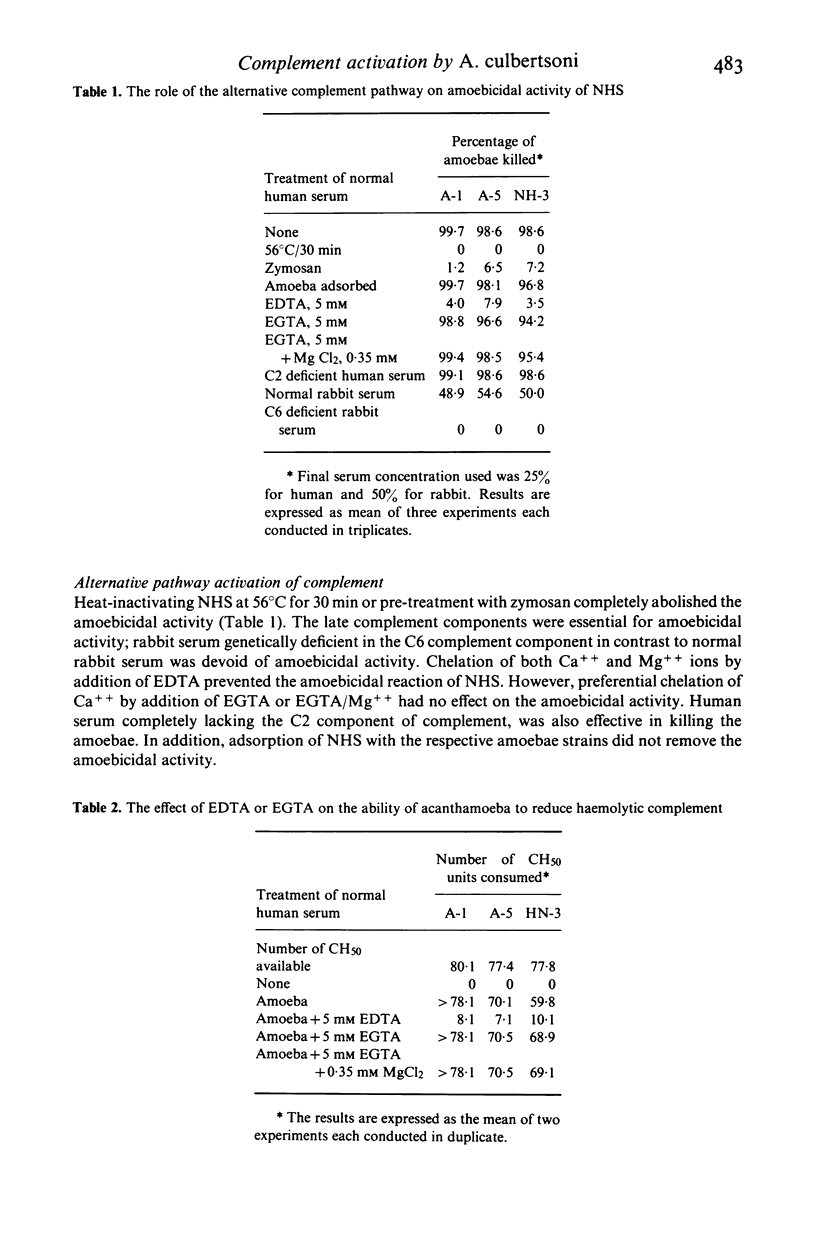
Normal human serum (NHS) contained an amoebicidal property for Acanthamoeba culbertsoni. Killing was quantitated by measuring the ability of the amoebae to undergo cell division subsequent to exposure to NHS, and also by microscopical examination. Plasma membrane disruption and extrusion of intracellular components occurred within 5-10 min following exposure to NHS. Adsorption of specific antibody did not remove the amoebicidal activity while heating serum at 56 degrees C/30 min or treatment with zymosan prevented the killing of A. culbertsoni. Haemolytic complement was consumed and C3 conversion occurred during the incubation of NHS with amoebae. Killing required the presence of the late complement components. The findings that (a) amoebae were killed in C2 deficient human serum and ethylene glycol tetra-acetic acid (EGTA), but not ethylenediamine tetra-acetic acid (EDTA) treated NHS; (b) haemolytic complement consumption, which occurred by incubating NHS with the amoebae, could be prevented by addition of EDTA, but not EGTA and (c) conversion of C3 occurred in the presence of EGTA, but not EDTA, indicated that activation of the alternative pathway of complement was involved. This may be of importance as a natural defence mechanism in humans against A. culbertsoni infections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter R. F. Description of a Naegleria sp. isolated from two cases of primary amoebic meningo-encephalitis, and of the experimental pathological changes induced by it. J Pathol. 1970 Apr;100(4):217–244. doi: 10.1002/path.1711000402. [DOI] [PubMed] [Google Scholar]
- Carter R. F. Primary amoebic meningo-encephalitis. An appraisal of present knowledge. Trans R Soc Trop Med Hyg. 1972;66(2):193–213. doi: 10.1016/0035-9203(72)90147-2. [DOI] [PubMed] [Google Scholar]
- Clarke H. G., Freeman T. Quantitative immunoelectrophoresis of human serum proteins. Clin Sci. 1968 Oct;35(2):403–413. [PubMed] [Google Scholar]
- Culbertson C. G. The pathogenicity of soil amebas. Annu Rev Microbiol. 1971;25:231–254. doi: 10.1146/annurev.mi.25.100171.001311. [DOI] [PubMed] [Google Scholar]
- Ferrante A. Experimental pneumonitis induced by Naegleria fowleri in mice. Trans R Soc Trop Med Hyg. 1981;75(6):907–908. doi: 10.1016/0035-9203(81)90450-8. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Activation of the alternative complement pathway by Torulopsis glabrata. Scand J Infect Dis. 1979;11(1):77–79. doi: 10.3109/inf.1979.11.issue-1.13. [DOI] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Holbrook T. W., Boackle R. J., Parker B. W., Vesely J. Activation of the alternative complement pathway by Naegleria fowleri. Infect Immun. 1980 Oct;30(1):58–61. doi: 10.1128/iai.30.1.58-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D., Olivecrona T. Composition of an amoeba plasma membrane. Biochem Biophys Res Commun. 1971 Oct 1;45(1):90–97. doi: 10.1016/0006-291x(71)90054-4. [DOI] [PubMed] [Google Scholar]
- Martinez J., Duma R. J., Nelson E. C., Moretta F. L. Experimental naegleria meningoencephalitis in mice. Penetration of the olfactory mucosal epithelium by Naegleria and pathologic changes produced: a light and electron microscope study. Lab Invest. 1973 Aug;29(2):121–133. [PubMed] [Google Scholar]
- Richards R. L., Gewurz H., Siegel J., Alving C. R. Interactions of C-reactive protein and complement with liposomes. II. Influence of membrane composition. J Immunol. 1979 Apr;122(4):1185–1189. [PubMed] [Google Scholar]
- Rowan-Kelly B., Ferrante A., Thong Y. H. Activation of complement by Naegleria. Trans R Soc Trop Med Hyg. 1980;74(3):333–336. doi: 10.1016/0035-9203(80)90092-9. [DOI] [PubMed] [Google Scholar]
- Sneiderman C. A., Wilson J. W. Effects of corticosteroids on complement and the neutrophilic polymorphonuclear leukocyte. Transplant Proc. 1975 Mar;7(1):41–48. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A. Alternative pathway of complement activation by Candida albicans. Aust N Z J Med. 1978 Dec;8(6):620–622. doi: 10.1111/j.1445-5994.1978.tb04850.x. [DOI] [PubMed] [Google Scholar]
- Thong Y. H. Primary amoebic meningoencephalitis: fifteen years later. Med J Aust. 1980 Apr 19;1(8):352–354. doi: 10.5694/j.1326-5377.1980.tb134919.x. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Simpson D. A., Müller-Eberhard H. J. Homozygous deficiency of the second component of complement presenting with recurrent bacterial meningitis. Arch Dis Child. 1980 Jun;55(6):471–473. doi: 10.1136/adc.55.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]