Abstract
The precise orchestration of synaptic differentiation is critical for efficient information exchange in the nervous system. The nerve–muscle synapse forms in response to agrin, which is secreted from the motor nerve terminal and induces the clustering of acetylcholine receptors (AChRs) and other elements of the postsynaptic apparatus on the subjacent muscle cell surface. In view of the highly restricted spatial localization and the plasticity of neuromuscular junctions, it seems likely that synapse formation and maintenance are regulated by additional, as-yet-unidentified factors. Here, we tested whether neurotrophins modulate the agrin-induced differentiation of postsynaptic specializations. We show that both brain-derived neurotrophic factor (BDNF) and neurotrophin-4 (NT-4) inhibit agrin-induced AChR clustering on cultured myotubes. Nerve growth factor and NT-3 are without effect. Muscle cells express full-length TrkB, the cognate receptor for BDNF and NT-4. Direct activation of this receptor by anti-TrkB antibodies mimicked the BDNF/NT-4 inhibition of agrin-induced AChR clustering. This BDNF/NT-4 inhibition is likely to be an intrinsic mechanism for regulating AChR clustering, because neutralization of endogenous TrkB ligands resulted in elevated levels of AChR clustering even in the absence of added agrin. Finally, high concentrations of agrin can occlude the BDNF/NT-4 inhibition of AChR clustering. These results indicate that an interplay between agrin and neurotrophins can regulate the formation of postsynaptic specializations. They also suggest a mechanism for the suppression of postsynaptic specializations at nonjunctional regions.
The formation, maintenance, and plasticity of synaptic connections is essential for the proper functioning of the nervous system. A hallmark of fast synapses is the precise spatial registration of the nerve terminal and postsynaptic apparatus. This alignment has been long appreciated in nerve–muscle synapses (1) and has also been demonstrated in a wide range of neuronal synapses (2). Synaptic structure also is tightly regulated: a large number of the synaptic connections initially formed in both the central nervous system (CNS) and the periphery are pruned by the process of synapse elimination. Some aspects of learning and memory also are likely to involve structural changes at synapses (3). Finally, unmatched pre- or postsynaptic specializations are rarely observed in mature muscle or the CNS.
Synapse formation is best understood at the neuromuscular junction. Mechanisms known to mediate its differentiation include neuregulins/ARIA (4), electrical activity (5), and agrin (6). Agrin plays an early and central role in nerve–muscle synapse formation. This extracellular matrix molecule is secreted from the nerve terminal and induces the clustering of acetylcholine receptors (AChRs) as well as the organization of other postsynaptic elements on the muscle cell surface. Targeted deletion experiments in mice have shown that agrin and its signaling receptor (muscle-specific kinase; MuSK) are essential for postsynaptic differentiation (7, 8). These experiments also revealed that agrin and MuSK are necessary for presynaptic apparatus formation and for the synapse-selective transcription of genes encoding AChR subunits. Finally, recombinant agrin presented extrasynaptically in denervated adult muscle can induce postsynaptic differentiation (9). Thus, agrin is necessary and in at least some aspects sufficient for inducing postsynaptic differentiation.
AChR clustering on the muscle cell surface is highly regulated. For example, ectopic postsynaptic specializations fail to form if a foreign nerve is presented extrasynaptically (10). Although extrajunctional AChR clusters are scarce in normal muscle, they rapidly accumulate following denervation (11, 12). Furthermore, during synapse elimination the postsynaptic apparatus is lost before nerve terminal withdrawal (13), suggesting that there are factors acting to disperse AChR clusters even in the continued presence of the nerve terminal. Together, these observations point to the existence of factors that modulate AChR clustering.
Neurotrophins are a family of neurotrophic factors first appreciated for their neuron-survival and neurite-outgrowth activities (14). The major class of receptors for these polypeptides is the Trk family of receptor tyrosine kinases. TrkA and TrkC are the primary receptors for nerve growth factor (NGF) and neurotrophin-3 (NT-3), respectively; TrkB serves as a receptor for both brain-derived neurotrophic factor (BDNF) and NT-4 (15, 16). Recent work has revealed an unexpectedly diverse range of neurotrophin activities (17), including a role in synaptic function and plasticity (18, 19). For example, overexpression of NT-4 by muscle potentiates neurotransmitter release from the motor neuron nerve terminal (20). In addition, Loeb and Fischbach (21) have shown that BDNF up-regulates neuregulin mRNA expression in motor neurons. Neurotrophins have also been implicated in visual cortex plasticity (22), dendritic differentiation (23), and long-term potentiation (18, 24). Despite these provocative findings, it has been difficult to sort out the cellular and molecular basis of these neurotrophin effects.
Here we have investigated whether neurotrophins regulate agrin-induced postsynaptic differentiation. We used the simple system of agrin-induced AChR clustering on cultured myotubes. Because no neurons are present in these cultures, it was possible to restrict the analysis to events occurring on the postsynaptic cell. We find that exogenous BDNF/NT-4 inhibits agrin-induced AChR clustering through a TrkB-dependent mechanism. Furthermore, our findings indicate that tonic inhibition by BDNF/NT-4 is an intrinsic mechanism for regulating the formation of postsynaptic specializations. These results suggest that the agrin pathway could be a target of neurotrophin-mediated synaptic plasticity.
MATERIALS AND METHODS
Cultures.
Chicken myotube cultures were prepared from pectoral muscle of embryonic day 11 (E11) embryos as described (25). Muscle cells were cultured on glass coverslips coated with poly-d-lysine and gelatin in minimum essential medium (alpha medium; GIBCO) supplemented with 10% horse serum, 2% chicken embryo extract, and 100 units/ml penicillin. Cultures were used 3–5 days after plating.
Recombinant Agrin.
A recombinant fragment of rat agrin12,4,8 corresponding to the C-terminal half of the molecule (26) was used in these experiments. This alternatively spliced form of agrin is expressed exclusively in neurons, including motor neurons, and is among the most potent for inducing AChR clustering on myotubes. A cDNA clone encoding this fragment (generously provided by M. Ferns, McGill University, Montreal) was produced in COS cells as described (27).
AChR Clustering Assays.
Myotubes were incubated with recombinant rat agrin for 12–16 hr. Increasing amounts of agrin were added to the myotube cultures to determine an initial concentration/effect curve. An agrin concentration of 20 pM yielded a half-maximal agrin stimulation, whereas a dose of 100 pM agrin yielded maximal agrin stimulation (see Fig. 4B). All test compounds were added at the time of agrin application. Rhodamine-labeled α-bungarotoxin was added to the culture medium 1 hr before fixation. Coverslips were allowed to air dry, mounted in Citifluor (Pella, Redding, CA), and viewed under epifluorescence optics with a Nikon Eclipse E-800 microscope. The number of AChR clusters ≥4 μm in their longest dimension were counted along 400-μm segments of myotube membrane. Ten myotube segments per coverslip were scored, and each experiment consisted of four coverslips per treatment. The number of experiments performed is reported as n. The P values were determined by using ANOVA and Newman–Keuls multiple-comparison test performed on the means.
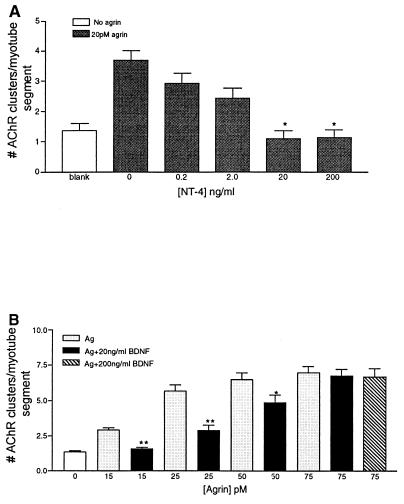
Figure 4.
Evidence that neurotrophins are acting through the agrin pathway. (A) Myotubes were stimulated with 20 pM agrin to induce AChR clustering in the presence of increasing concentrations of NT-4. NT-4 showed a dose-dependent inhibition from 0.2 ng/ml to 20 ng/ml. Increasing the concentration above 20 ng/ml had no additional effect on the number of AChR clusters. (B) High concentrations of agrin occlude BDNF inhibition of agrin-induced AChR clustering. Myotubes were treated with increasing concentrations of agrin (Ag) in the presence or absence of BDNF (20 ng/ml), and the number of AChRs clustered was determined. BDNF significantly inhibits AChR clustering at all but the highest agrin concentration. High concentrations of BDNF (200 ng/ml) did not significantly decrease agrin-induced AChR clustering at high agrin concentrations. (∗, P < 0.01; ∗∗, P < 0.001). Values shown are mean ± SEM.
Antibodies.
Rabbit anti-chicken TrkB and rabbit anti-chicken TrkC were generous gifts of F. Lefcort (Montana State University, Bozeman, MT) and L. Reichardt (University of California, San Francisco). Each of these antisera specifically activates its respective Trk as shown by their ability to sustain DRG cells in culture (28, 29). This TrkB antibody binds to neither TrkA nor TrkC, and the monomeric fab fragment neither activates TrkB nor sustains survival of DRG cells in culture (29). The TrkB-Fc fusion and the irrelevant Fc fusion were provided by D. Shelton (Genentech Inc., South San Francisco, CA).
Immunohistochemistry.
Myotubes grown in culture for 3 days were fixed with 4% paraformaldehyde. Nonspecific binding sites were blocked by incubation in medium containing 10% horse serum and 1% goat serum followed by incubation with a polyclonal antibody to either TrkB (29) or an irrelevant antibody (both at 1 μg/ml) for 12 hr at 4°C. Bound antibody was detected with a Cy3-conjugated goat anti-rabbit IgG (1:500; Jackson ImmunoResearch). Coverslips were mounted in Citifluor and visualized under rhodamine optics.
Analysis of TrkB mRNA Expression in Myotubes.
For RNA extraction, chicken myotube cultures were prepared as described above but were grown in 10-cm culture dishes for 5–7 days. Myotubes were trypsinized and the RNA was extracted (RNeasy kit, Qiagen, Chatsworth, CA). Myotube RNA was reversed-transcribed with Moloney-murine leukemia virus (M-MLV) reverse transcriptase and random primers (Gibco) and the resultant cDNA was PCR-amplified by using primers specific for the full-length, kinase-containing form of TrkB (30). The forward upstream primer is 5′-AAA ATA CAT GTT ATC AAT CA-3′ and the downstream primer is 5′-ACT CCT CAC TGC TAC CAA 3-′. The product of this PCR reaction was amplified further with nested primers: upstream 5′-GGA CAA CCC TAC CCA CCT GA-3′ and downstream 5′-TTC TCC CAG TTC TCT TTT GA-3′.
An oligo(dT)-primed cDNA library from cultured chicken myotube mRNA was constructed and ligated into the pCMX vector using the methods described by Davis et al. (31). This library was used in the same nested PCR experiments described above.
RESULTS
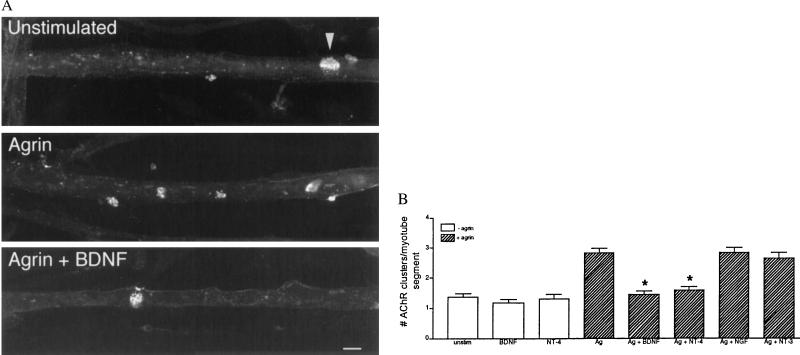
We first asked whether exogenously applied neurotrophins influence agrin-induced AChR clustering on myotubes (Fig. 1). On untreated myotubes, most AChRs are diffusely distributed on the cell surface, whereas some are arrayed in spontaneous clusters called hot spots. Addition of agrin induced the aggregation of the diffusely arrayed AChRs into clusters. However, agrin failed to induce AChR clustering in the presence of added BDNF or NT-4 (Fig. 1). Two other neurotrophins, NGF and NT-3, did not affect the levels of agrin-induced AChR clusters, even when used at 10-fold higher concentrations. None of the neurotrophins tested altered the level of spontaneous AChR clusters (Fig. 1B; data not shown). Thus, BDNF and NT-4 inhibit agrin-mediated differentiation of postsynaptic specializations.
Figure 1.
Exogenous BDNF and NT-4 inhibit agrin-mediated AChR clustering. (A) Cultured myotubes were grown for 3 days and then incubated for 12 hr in media only (unstimulated), media plus agrin (20 pM), or agrin and BDNF (20 ng/ml) as indicated. AChRs were labeled with rhodamine α-bungarotoxin. In unstimulated myotubes, the AChRs are diffusely distributed, with only occasional spontaneous clusters observed (arrowhead). The addition of agrin to the myotubes induced an increase in the number of AChR clusters. In the presence of BDNF, agrin failed to induce AChR clusters. (Bar = 20 μm.) (B) Sensitivity of agrin-induced AChR clustering to various neurotrophins. Myotubes were treated with the indicated neurotrophin in the presence (hatched bars) or absence (open bars) of 20 pM agrin (Ag). BDNF and NT-4 (20 ng/ml) inhibited agrin-induced AChR clustering (n = 4; ∗, P < 0.001) but had no effect on the number of spontaneous clusters (n = 3). The level of agrin-induced AChR clustering was unaffected when NGF or NT-3 (20 ng/ml) was added (n = 4). Mean ± SEM.
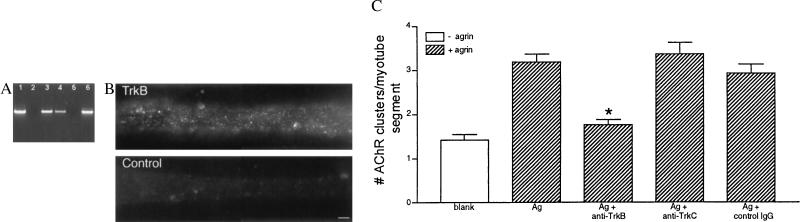
BDNF and NT-4 are both ligands of TrkB (32–35). Therefore, we next asked whether the BDNF/NT-4 inhibition of AChR clustering we observed was mediated by TrkB receptors on the myotube surface. To demonstrate the presence of message encoding full-length TrkB in these myotubes, we performed RT-PCR on RNA isolated from them (30). Fig. 2A shows the message for the tyrosine kinase-containing form of TrkB is expressed in cultured myotubes. Sequences encoding full-length TrkB also were detected in a chicken myotube cDNA library. These results are in agreement with previous studies that have shown TrkB mRNA expression in skeletal muscle (36, 37). Immunohistochemistry with a TrkB-specific antibody demonstrated that cultured chicken myotubes express TrkB on their surface (Fig. 2B). To determine whether TrkB activation is sufficient to mediate inhibition of AChR clustering, we directly activated this receptor by using antibody-mediated dimerization (28, 29, 38). Importantly, this treatment also inhibited agrin-induced AChR clustering (Fig. 2C). In contrast, incubation with anti-TrkC or an irrelevant IgG had no effect, even at 5-fold higher concentrations. In addition, activation of the fibroblast growth factor receptor tyrosine kinase by the addition of basic fibroblast growth factor (30 ng/ml, GIBCO) did not inhibit agrin-induced AChR clustering (data not shown), indicating that the neurotrophins are not likely to be acting by sequestering signaling molecules used by receptor tyrosine kinase activation. Together, these data indicate that BDNF and NT-4 mediate the inhibition of AChR clustering by a TrkB-dependent pathway.
Figure 2.
BDNF/NT-4 inhibition of AChR clustering is mediated by TrkB receptors. (A) The mRNA-encoding full-length TrkB is present in cultured chicken myotubes. Nested PCR performed on a clone of full-length chicken TrkB produced a product of approximately 570 bp (lane 1). Nested PCR performed on two concentrations of a cDNA library made from chicken myotubes (lanes 3 and 4) or reverse transcription–PCR on RNA isolated from chicken myotubes in culture (lane 6) also produced an identical sized product. Lanes 2 and 5 had water substituted for DNA in the nested PCR reaction to control for contamination. (Bar = 5 μm.) (B) TrkB expression in myotubes. Cultured myotubes were fixed and labeled with either anti-TrkB antibody or an irrelevant IgG followed by an anti-rabbit-IgG secondary antibody conjugated to Cy3. TrkB immunoreactivity is distributed in a finely punctate pattern along the surfaces of the myotube. (C) Sensitivity of agrin-induced AChR clustering to Trk receptor activation. Myotubes were incubated (12 hr) with 10 μg/ml anti-TrkB, anti-TrkC, or an irrelevant IgG (control IgG) in the presence of 20 pM agrin (Ag; hatched bars). At this concentration, both anti-Trk antibodies activate their cognate receptors (28, 29). Activation of TrkB inhibited agrin-induced clustering (n = 4; ∗, P < 0.001). Treatment with anti-TrkC (10 μg/ml) or an irrelevant IgG had no effect on agrin-mediated AChR cluster number (n = 3). Mean ± SEM.
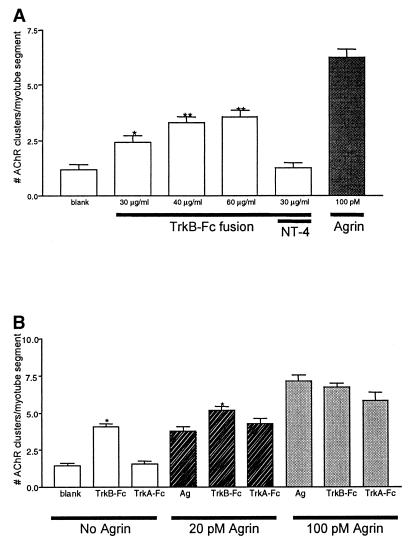
The results presented above indicate that exogenous BDNF/NT-4 blocks agrin-induced AChR clustering. To determine whether such an inhibitory pathway is an intrinsic feature of muscle cells, we asked whether TrkB ligands secreted by myotubes regulate AChR cluster formation. Previous work has shown that NT-4 is expressed by muscle (39). To neutralize endogenous neurotrophins, we incubated the myotubes with TrkB-Fc, a fusion protein combining the extracellular, ligand-binding domain of TrkB with an IgG heavy chain. TrkB-Fc thus acts as a scavenger that binds to extracellular BDNF or NT-4. In these experiments, neither agrin nor neurotrophins were added to the cultures. Treatment with TrkB-Fc resulted in a dose-dependent increase in the number of AChR clusters, which peaked at a two-fold increase over the number of spontaneous clusters (Fig. 3A). The increase in AChR clustering induced by the TrkB-Fc fusion was blocked by addition of excess NT-4 (200 ng/ml). In addition, TrkB-Fc potentiates the number of AChR clusters formed in response to submaximal concentrations of agrin (Fig. 3B) but had no affect on the number of clusters formed in response to maximal agrin concentrations. In contrast, a TrkA-Fc fusion or normal human IgG had no effect (Fig. 3B; data not shown). Together, these data suggest that muscle-derived neurotrophins, acting in an autocrine fashion, limit the formation of AChR clusters. These findings also indicate that the neurotrophin-induced inhibition is influencing the agrin signaling pathway.
Figure 3.
Endogenous TrkB ligands regulate AChR cluster formation. (A) Myotubes were incubated with increasing concentrations of TrkB-Fc fusion (30–60 μg/ml) to neutralize endogenous TrkB ligands secreted by the myotubes. The number of AChR clusters increased in cultures incubated with TrkB-Fc but did not attain the level of AChR clustering seen with a saturating amount of agrin. Addition of NT-4 (200 ng/ml) was able to reverse the TrkB-Fc induced increase in AChR clusters (n = 3; ∗, P < 0.01; ∗∗, P < 0.001). (B) Myotubes were treated with TrkB-Fc fusion (or control TrkA-Fc fusion) either alone or in the presence of agrin concentrations that induced half-maximal (20 pM) or maximal (100 pM) levels of AChR clustering. TrkB-Fc fusion potentiated the agrin-induced clustering seen with 20 pM agrin, but had no effect on the number of clusters formed in response to maximal concentrations of agrin. No significant change in AChR clustering was observed when TrkA-Fc fusions were used. (n = 3; ∗, P < 0.001). Values shown are mean ± SEM.
Muscle cells in vivo are undoubtedly exposed to both neural agrin and to BDNF/NT-4. To assess the relative strengths of these stimulatory and inhibitory signals for AChR clustering, we determined the respective effects of increasing concentrations of agrin and BDNF. In myotube cultures treated with a half-maximal agrin concentration (20 pM), NT-4 caused a dose-dependent inhibition of the number of AChR clusters formed (Fig. 4A). However, the concentration of neurotrophin that yielded a complete inhibition of clustering could be occluded when the agrin concentration was increased (Fig. 4B). In addition, a 10-fold greater concentration of BDNF (200 ng/ml) had no effect on the number of clusters formed in response to maximal agrin concentrations (75 pM). Similarly, the anti-TrkB antibody-mediated inhibition also was overcome by increasing concentrations of exogenously applied agrin (data not shown). Thus, neurotrophins modulate agrin-induced AChR clustering. However, at sufficiently high concentrations agrin is the dominant influence in the formation of postsynaptic specializations on these cultured chicken myotubes.
DISCUSSION
The findings presented here show that neurotrophic factors regulate postsynaptic differentiation on muscle cells. We used several independent approaches to test this hypothesis, and they yielded a remarkably coherent answer. A survey of the four major neurotrophins showed that only BDNF and NT-4 influenced AChR clustering. We then demonstrated that cultured muscle cells express mRNA encoding full-length TrkB and that TrkB is present on the myotube cell surface. Direct activation of the TrkB receptor by a TrkB-specific antibody mimicked the effect of added BDNF/NT-4. Importantly, the muscle cells themselves use neurotrophins to regulate the clustering of AChRs on their surface: neutralization of endogenous TrkB ligands results in an increase in the level of AChR aggregates on the cell. Finally, we showed that high concentrations of agrin can occlude the neurotrophin inhibition observed on these myotubes, suggesting that an interplay of agrin stimulation and neurotrophin inhibition could serve to regulate postsynaptic differentiation. The implications of these findings are discussed below.
A general conclusion of these findings is that factors known to be present in muscle can negatively regulate AChR clustering. It is well established that an activity-dependent pathway down-regulates extrasynaptic AChR synthesis (1); however, mechanisms that might suppress their clustering have not been defined. Nonetheless, a number of observations have anticipated the existence of such inhibitory pathways. For example, a foreign motor nerve presented extrasynaptically to an innervated muscle fails to induce postsynaptic differentiation. In addition, extrasynaptic AChR clusters are rarely observed in developing muscle in vivo (40–42) even though there are high levels of extrajunctional AChRs on the myofibers at these times (43). The results presented here suggest that neurotrophins could act to suppress extrasynaptic AChR clustering. Interestingly, muscle-derived NT-4 levels rise after innervation and decrease on denervation (39); this decrease in NT-4 could contribute to the ability of denervated muscle to accept extrasynaptic innervation.
BDNF/NT-4 could also play a role in postsynaptic differentiation. The release of agrin by the nerve terminal and its binding to the extracellular matrix is undoubtedly a key mechanism in ensuring that postsynaptic specializations are arrayed opposite the presynaptic apparatus. The dominance of high agrin concentrations over neurotrophin inhibition observed here is consistent with this idea. However, synapses undergo extensive remodeling. During synapse elimination, the postsynaptic apparatus is removed from underneath one of two neighboring nerve terminals. Furthermore, the remaining postsynaptic apparatus is reshaped from its original plaque-like configuration to the elaborately branched structure characteristic of a mature endplate. Neurotrophins could potentially play a role in one or both of these processes.
The neurotrophin-mediated regulation of AChR clustering is likely to involve an interaction between the signaling pathways for neurotrophins and for agrin. The BDNF/NT-4-mediated inhibition is probably not the result of regulation of gene expression, because we observe similar inhibition of agrin-induced AChR clustering in short-term (4-hr) experiments (unpublished observations). Many steps in the agrin pathway, including activation of the agrin signaling receptor MuSK (7, 44), recruitment of rapsyn (45, 46), intracellular calcium fluxes (47), and tyrosine phosphorylation of AChRs (48) have been elucidated. It will be of interest to determine which of these steps may be modulated by the action of neurotrophins. Notably, the muscle cell could potentially attenuate (or potentiate) the agrin clustering signal through regulating the activity and/or number of TrkB receptors. It will also be important to characterize the AChR clustering induced by neutralization of endogenous BDNF/NT-4 in the absence of added agrin. The trigger for such spontaneous clusters is not known, but their formation often requires the MuSK-based signaling pathway (49).
Neurotrophins are emerging as key modulators of synaptic differentiation and plasticity in both muscle (20, 21) and the CNS (18, 50–52). NT-4 overexpression in muscle cells potentiates synaptic transmission by increasing transmitter release from the nerve terminal (20). In addition, Wang and Poo (20) also demonstrated the ability of NT-4 to mediate AChR gating properties, presumably through activation of TrkB receptors on the muscle. However, the molecular basis by which neurotrophins regulate synapse formation and stability, particularly with respect to the postsynaptic apparatus, is unknown. The results presented here indicate that neurotrophins modulate the agrin signaling pathway that leads to the formation of postsynaptic specializations. A model for the role of BDNF/NT-4 in regulating synaptic plasticity is depicted in Fig. 5. This model takes into account the role of these neurotrophins in postsynaptic differentiation presented here as well as work by others showing that these factors potentiate neurotransmitter release (20) and stimulate the production of ARIA (21). Thus, muscle-derived BDNF/NT-4 can regulate synaptic differentiation by activation of TrkB receptors on both the nerve terminal and the muscle.
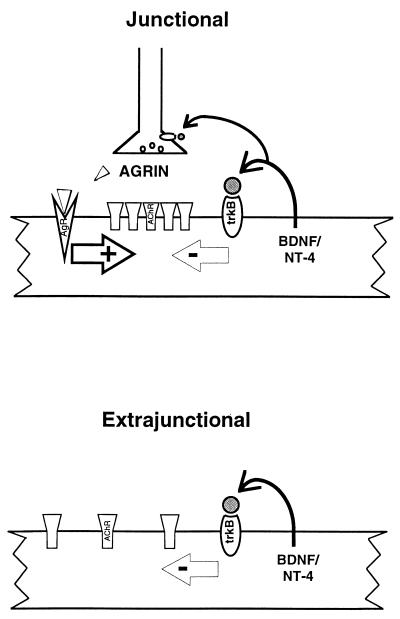
Figure 5.
A model of how agrin and neurotrophins could work in concert to establish and maintain proper postsynaptic architecture. Agrin released by the motor neuron must overcome the intrinsic inhibition of BDNF/NT-4 to cluster AChRs. At the site of synaptic contact (Upper) agrin is at sufficiently high concentration to occlude this inhibition. Extrajunctionally (Lower), TrkB-mediated inhibition of clustering is the dominant force. Work by others indicates that BDNF/NT-4 released by muscle could also regulate synaptic function through activation of presynaptic TrkB receptors (see Discussion). This model provides a mechanism for the restriction of postsynaptic specializations to subsynaptic regions.
Could the interaction between BDNF/NT-4 and agrin signaling provide insights into synapse formation and/or plasticity in the brain? BDNF has clear effects on synaptic function in the hippocampus (18, 24, 52, 53). In addition, agrin is expressed at synapses on cultured hippocampal neurons (D.G.W. and J.R.F., unpublished observations). At present, neither the mechanism of action of BDNF nor the role of agrin at CNS synapses is known. However, the present findings raise the possibility that an interplay between agrin and BDNF could regulate synaptic structure or function in the CNS.
Acknowledgments
We thank P. Zabel for excellent technical support; F. Lefcort, D. Shelton, and L. Reichardt for anti-Trk reagents; T. Large and K. Boeshore for chicken TrkB sequence and primer sequence; D. Mendis, K. Deyst, M. Bowe, and M.-A. Abbott for comments on the manuscript. D.G.W. was supported by a postdoctoral fellowship from the National Institutes of Health (National Research Service Award NS10343). This work was supported by the Muscular Dystrophy Association and the National Institutes of Health (HD23924 and MH53571).
ABBREVIATIONS
- AChR
acetylcholine receptor
- BDNF
brain-derived neurotrophic factor
- NT-4
neurotrophin-4
- MuSK
muscle-specific kinase
- CNS
central nervous system
- NT
neurotrophin
References
- 1.Hall, Z. W. & Sanes, J. R. (1993) Neuron10, Suppl., 99–121.
- 2.Peters A, Palay S L, Webster Hd. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. 3rd Ed. New York: Oxford University Press; 1991. [Google Scholar]
- 3.Bailey C H, Kandel E R. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 4.Carraway KL, III, Burden S J. Curr Opin Neurobiol. 1995;5:606–612. doi: 10.1016/0959-4388(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 5.Goldman D, Brenner H R, Heinemann S. Neuron. 1988;1:329–333. doi: 10.1016/0896-6273(88)90081-5. [DOI] [PubMed] [Google Scholar]
- 6.McMahan U J. Cold Spring Harbor Symp Quant Biol. 1990;50:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- 7.DeChiara T M, Bowen D C, Valenzuela D M, Simmons M V, Poueymirov W T, Thomas S, Kinetz E, Compton D L, Rojas E, Park J S, et al. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 8.Gautam M, Noakes P G, Moscoso L, Rupp F, Scheller R H, Merlie J P, Sanes J R. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 9.Cohen I, Rimer M, Lomo T, McMahan U J. Mol Cell Neurosci. 1997;9:237–253. doi: 10.1006/mcne.1997.0623. [DOI] [PubMed] [Google Scholar]
- 10.Lomo T, Mirsky R, Pockett S. In: Neuromuscular Diseases. Serratrice G, Cros D, Desnuelle C, editors. New York: Raven; 1984. pp. 393–399. [Google Scholar]
- 11.Ishikawa Y, Arakaki A, Shimizu N, Ibaraki K, Tanaka S. Dev Biol. 1988;125:115–126. doi: 10.1016/0012-1606(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 12.Peng H B. J Neurosci. 1986;6:581–589. doi: 10.1523/JNEUROSCI.06-02-00581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balice-Gordon R J, Chua C K, Nelson C C, Lichtman J W. Neuron. 1993;11:801–815. doi: 10.1016/0896-6273(93)90110-d. [DOI] [PubMed] [Google Scholar]
- 14.Levi-Montalcini R. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 15.Meakin S O, Shooter E M. Trends Neurosci. 1992;15:323–331. doi: 10.1016/0166-2236(92)90047-c. [DOI] [PubMed] [Google Scholar]
- 16.Snider W. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 17.Elde F F, Lowenstein D H, Reichardt L F. Exp Neurol. 1993;121:200–214. doi: 10.1006/exnr.1993.1087. [DOI] [PubMed] [Google Scholar]
- 18.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 19.Lohof A M, Ip N Y, Poo M-M. Nature (London) 1993;363:350–352. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 20.Wang X-H, Poo M-M. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 21.Loeb J A, Fischbach G D. J Neurosci. 1997;17:1416–1424. doi: 10.1523/JNEUROSCI.17-04-01416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cellerino A, Maffei L. Prog Neurobiol. 1996;49:53–71. doi: 10.1016/0301-0082(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 23.McAllister A K, Lo D C, Katz L C. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 24.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nastuk M A, Lieth E, Ma J, Cardasis C A, Moynihan E B, McKechnie B A, Fallon J R. Neuron. 1991;7:807–818. doi: 10.1016/0896-6273(91)90283-6. [DOI] [PubMed] [Google Scholar]
- 26.Ferns M J, Campanelli J T, Hoch W, Scheller R H, Hall Z. Neuron. 1993;11:491–502. doi: 10.1016/0896-6273(93)90153-i. [DOI] [PubMed] [Google Scholar]
- 27.O’Toole J J, Deyst K A, Bowe M A, Nastuk M A, McKechnie B A, Fallon J R. Proc Natl Acad Sci USA. 1996;93:7369–7374. doi: 10.1073/pnas.93.14.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefcort F, Clary D O, Rusoff A C, Reichardt L F. J Neurosci. 1996;16:3704–3713. doi: 10.1523/JNEUROSCI.16-11-03704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Bartheld C S, Williams R, Lefcort F, Clary D O, Reichardt L F, Bothwell M. J Neurosci. 1996;16:2995–3008. doi: 10.1523/JNEUROSCI.16-09-02995.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garner A S, Menegay H J, Boeshore K L, Xie X-Y, Voci J M, Johnson J E, Large T H. J Neurosci. 1996;16:1740–1752. doi: 10.1523/JNEUROSCI.16-05-01740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis S, Aldrich T H, Vaenzuela D M, Wong V, Furth M E, Squinto S P, Yancopolous G D. Science. 1991;253:59–63. doi: 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- 32.Squinto S P, Stitt T N, Aldrich T H, Davis S, Bianes S M, Radziejewski C, Glass D J, Masiakowski P, Furth M E, Valenzuela D M, Yancopoulos G D. Cell. 1991;65:885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- 33.Glass D J, Yancopoulos G D. Trends Cell Biol. 1993;3:262–268. doi: 10.1016/0962-8924(93)90054-5. [DOI] [PubMed] [Google Scholar]
- 34.Barbacid M. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 35.Bothwell M. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 36.Shelton D L, Sutherland J, Gripp J, Camerato T, Armanini M P, Phillips H S, Carroll K, Spencer S D, Levinson A D. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto M, Sobue G, Yamamoto K, Terao S, Mitsuma T. Neurochem Res. 1996;21:929–938. doi: 10.1007/BF02532343. [DOI] [PubMed] [Google Scholar]
- 38.Jing S, Tapley P, Barbacid M. Neuron. 1992;9:1067–1079. doi: 10.1016/0896-6273(92)90066-m. [DOI] [PubMed] [Google Scholar]
- 39.Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez C F. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- 40.Fallon J R, Gelfman C E. J Cell Biol. 1989;108:1527–1535. doi: 10.1083/jcb.108.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen M W, Jacobson C, Godfrey E W, Campbell K P, Carbonetto S. J Cell Biol. 1995;129:1093–1101. doi: 10.1083/jcb.129.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dahm L M, Landmesser L T. J Neurosci. 1991;11:238–255. doi: 10.1523/JNEUROSCI.11-01-00238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burden S. Dev Biol. 1977;57:317–329. doi: 10.1016/0012-1606(77)90218-4. [DOI] [PubMed] [Google Scholar]
- 44.Glass D J, Bowen D C, Stitt T N, Radziejewski C, Bruno J, Ryan T E, Gies D R, Shah S, Mattson K, Burden S J, et al. Cell. 1996;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- 45.Phillips W D, Kopta C, Blount P, Gardner P D, Steinbach J H, Merlie J P. Science. 1991;251:568–570. doi: 10.1126/science.1703661. [DOI] [PubMed] [Google Scholar]
- 46.Gautam M, Noakes P G, Mudd J, Nichol M, Chu G C, Sanes J R, Merlie J P. Nature (London) 1995;377:232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- 47.Megeath L J, Fallon J R. J Neurosci. 1998;18:672–678. doi: 10.1523/JNEUROSCI.18-02-00672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace B G, Qu Z, Huganir R L. Neuron. 1991;6:869–878. doi: 10.1016/0896-6273(91)90227-q. [DOI] [PubMed] [Google Scholar]
- 49.Glass D J, DeChiara T M, Bowen D C, Stitt T N, Radziejewski C, Bruno J, Ryan T E, Gies D R, Shah S, Burdon S S, et al. Soc Neurosci Abstr. 1996;22:1476. [Google Scholar]
- 50.Lo D C. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 51.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 52.Levine E S, Dreyfus C F, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patterson S L, Abel T, Deuel T A, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]