Abstract
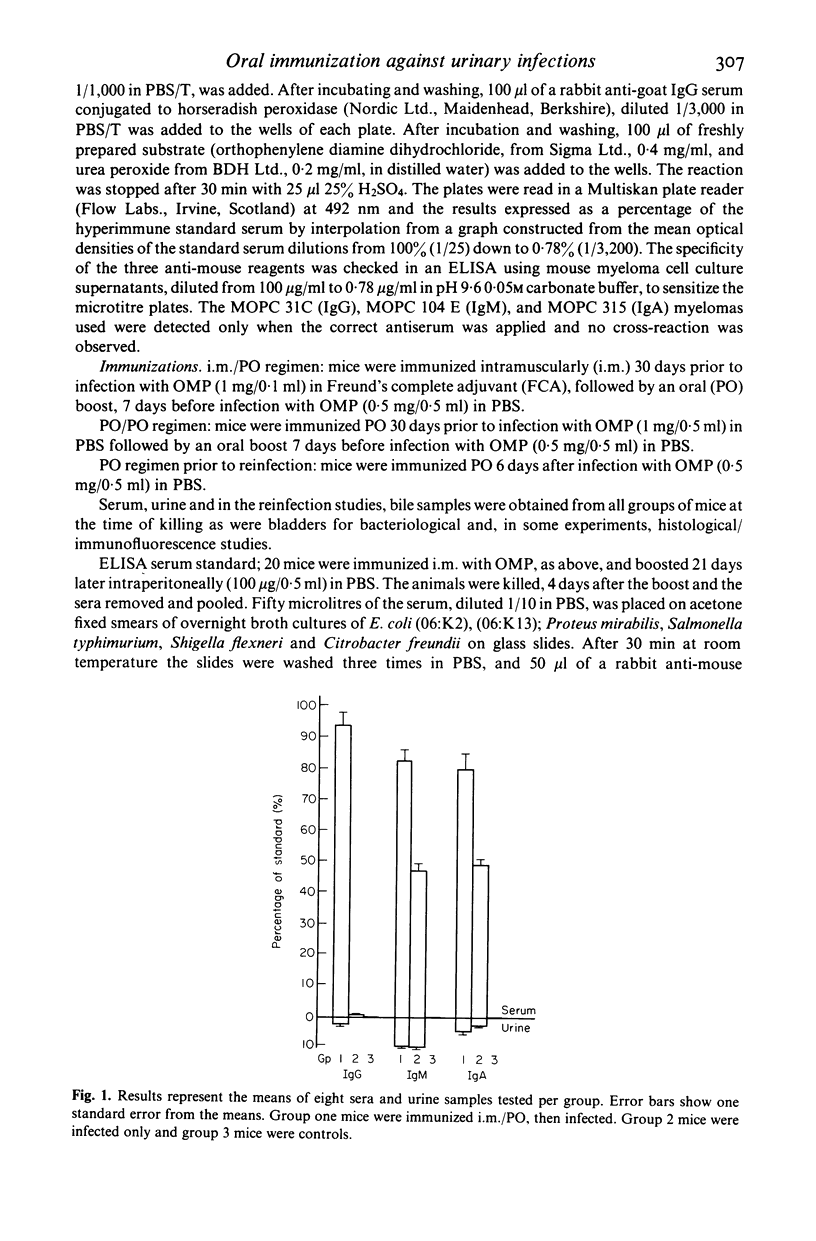
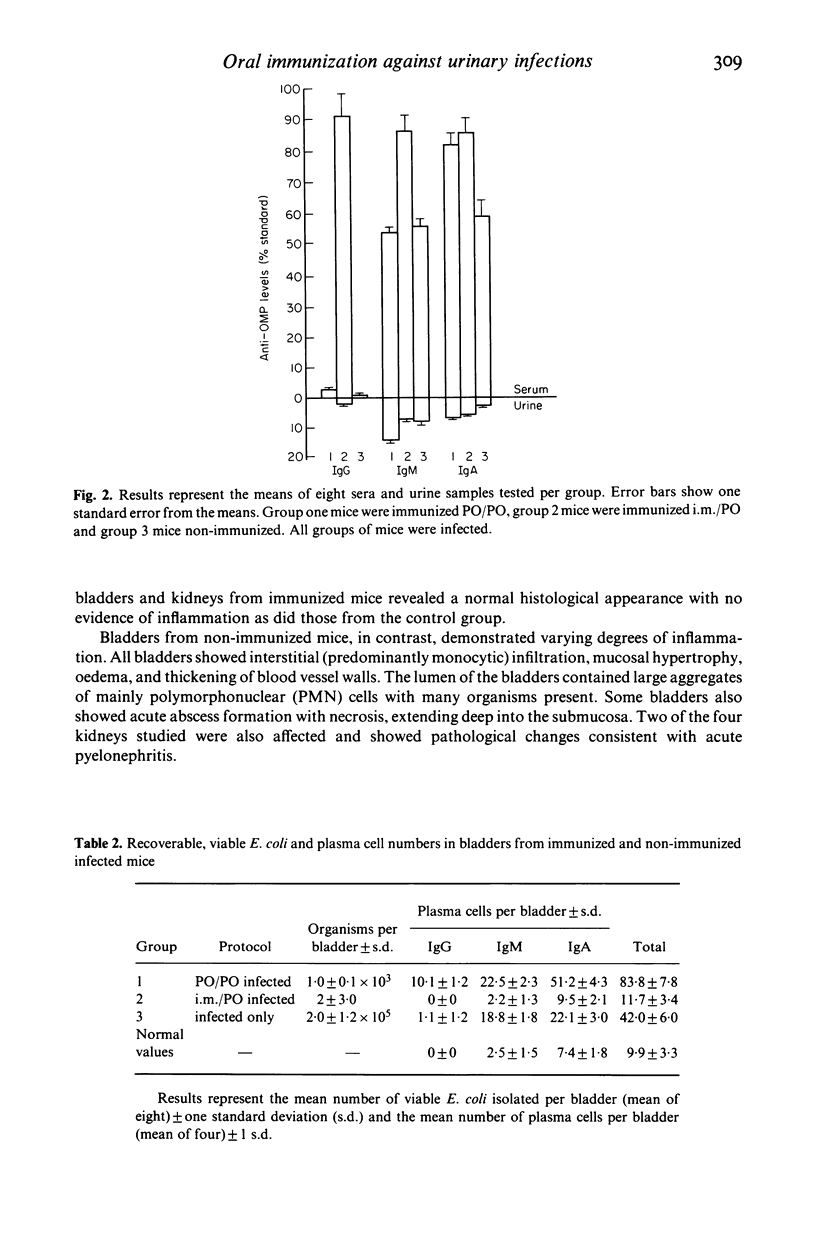
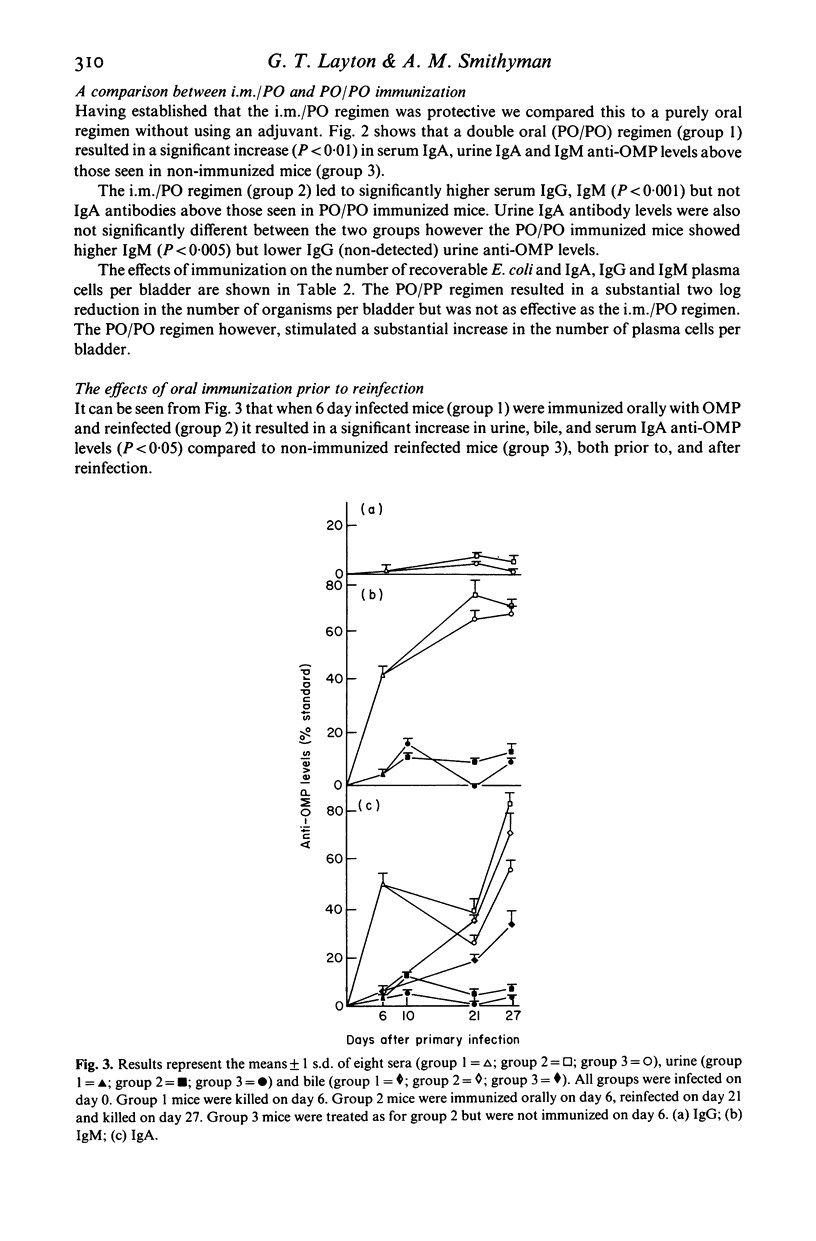
A double oral immunization (PO/PO) with an outer membrane protein (OMP) from a human uropathogenic strain of Escherichia coli, resulted in the partial protection of mice infected per urethrally with the same strain. Complete protection was achieved by immunizing with OMP in Freund's complete adjuvant (FCA), intramuscularly (i.m.), followed by an oral boost (i.m./PO). The PO/PO protocol stimulated mainly local urinary antibody synthesis, particularly IgA, whilst the i.m./PO regimen resulted in the appearance of both serum and urine antibodies. A single dose of OMP, 6 days after infection, rendered the mice resistant to reinfection, in contrast to non-immunized mice, and led to a significant increase in urine, serum and bile IgA anti-OMP levels. Our results confirm previous reports that the urinary tract forms part of the common mucosal immune system and provides further evidence for immunological memory in mucosal immunity. These results also demonstrate that our OMP preparation is a highly effective immunizing antigen, and that such preparations may be suitable as oral vaccines against urinary tract infection in humans.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew E., Hall J. G. IgA antibodies in the bile of rats. II. Evidence for immunological memory in secretory immunity. Immunology. 1982 Jan;45(1):177–182. [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Lefrancier P., Choay J., Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2472–2475. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly R., Waldman R. H. Local immunity and local immune responses. Prog Allergy. 1980;27:1–68. [PubMed] [Google Scholar]
- Hand W. L., Smith J. W., Miller T. E., Barnett J. A., Sanford J. P. Immunoglobulin synthesis in lower urinary tract infection. J Lab Clin Med. 1970 Jan;75(1):19–29. [PubMed] [Google Scholar]
- Jackson G. D., Lemaître-Coelho I., Vaerman J. P., Bazin H., Beckers A. Rapid disappearance from serum of intravenously injected rat myeloma IgA and its secretion into bile. Eur J Immunol. 1978 Feb;8(2):123–126. doi: 10.1002/eji.1830080210. [DOI] [PubMed] [Google Scholar]
- Kuriyama M. [The study of urinary secretory IgA. (I) Its localization in the urinary tract (author's transl)]. Nihon Hinyokika Gakkai Zasshi. 1979 Oct;70(10):1129–1144. doi: 10.5980/jpnjurol1928.70.10_1129. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marx J. L. Vaccinating with bacterial pili. Science. 1980 Sep 5;209(4461):1103–1106. doi: 10.1126/science.6105710. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- Nagura H., Nakane P. K., Brown W. R. Translocation of dimeric IgA through neoplastic colon cells in vitro. J Immunol. 1979 Nov;123(5):2359–2368. [PubMed] [Google Scholar]
- Orlans E., Peppard J., Reynolds J., Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978 Feb 1;147(2):588–592. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Sircar B. K. Immunity to experimental cholera. III. Enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977 Jun;135(6):888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- Poxton I. R. Serological identification of Bacteroides species by an enzyme-linked immunosorbent assay. J Clin Pathol. 1979 Mar;32(3):294–298. doi: 10.1136/jcp.32.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinno J., Golecki J. R., Mayer H. Localization of enterobacterial common antigen:immunogenic and nonimmunogenic enterobacterial common antigen-containing Escherichia coli. J Bacteriol. 1980 Feb;141(2):814–821. doi: 10.1128/jb.141.2.814-821.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg-Edén C., Svennerholm A. M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1978 Dec;22(3):790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi T. B., Jr, Larson L., Challacombe S., McNabb P. Mucosal immunity: The origin and migration patterns of cells in the secretory system. J Allergy Clin Immunol. 1980 Jan;65(1):12–19. doi: 10.1016/0091-6749(80)90171-2. [DOI] [PubMed] [Google Scholar]
- Virella G., Montgomery P. C., Lemaitre-Coelho I. M. Transport of oligomeric IgA of systemic origin into external secretions. Adv Exp Med Biol. 1978;107:241–251. doi: 10.1007/978-1-4684-3369-2_29. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- van der Bosch J. F., Verboom-Sohmer U., Postma P., de Graaff J., MacLaren D. M. Mannose-sensitive and mannose-resistant adherence to human uroepithelial cells and urinary virulence of Escherichia coli. Infect Immun. 1980 Jul;29(1):226–233. doi: 10.1128/iai.29.1.226-233.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



