Abstract
The nature and histological pattern of the cutaneous infiltrates of 17 leprosy patients in reversal reactions (Type I) and erythema nodosum leprosum (Type II, ENL) were compared with tissues from 18 non-reactional borderline leprosy (BT, BL) and lepromatous leprosy (LL) patients using monoclonal antibodies and immunofluorescence. Reactional BT lesions showed a mild increase in OKT11+ pan T cells as compared to non-reactional tissues and a significant influx of OKT8+ (suppressor/cytotoxic) cells which were peripherally localized in the lymphocyte mantle surrounding the epithelioid cells. The Leu 3a+ (helper/inducer) cells were scattered amongst the lymphocytes and macrophages. The mean ratio (+/- s.d.) of Leu 3a+/OKT8+ cells was 1.88 +/- 0.64 in Type I BT reactions as compared to 2.95 +/- 0.95 in BT lesions. In contrast, lesions of BL reversal reactions and ENL showed a more marked increase in pan T cells with a preponderance of the helper/inducer subset, Leu 3a+/OKT8+ ratio being 2.26 +/- 0.61 and 0.93 +/- 0.57 in BL reactional and non-reactional lesions, respectively. Interestingly, this increase in the numbers of the T cells reached levels observed in BT lesions. The distribution pattern of OKT8+ cells was similar to Leu 3a+, both being diffusely scattered amongst the bacilli laden macrophages. Ia like antigens were present in all granulomas and were abundant on lymphocytes and macrophages and less conspicuous on epithelioid cells. T6+ Langerhans cells were uniformly increased in all reactional lesions. It would appear that the changes observed in both Type I and Type II reactions are similar in the lepromatous group of patients. They differ significantly from the BT reversal reaction in terms of the dominant T cell subset and the microanatomical distribution of the OKT8+ cells in the lesions.
Full text
PDF

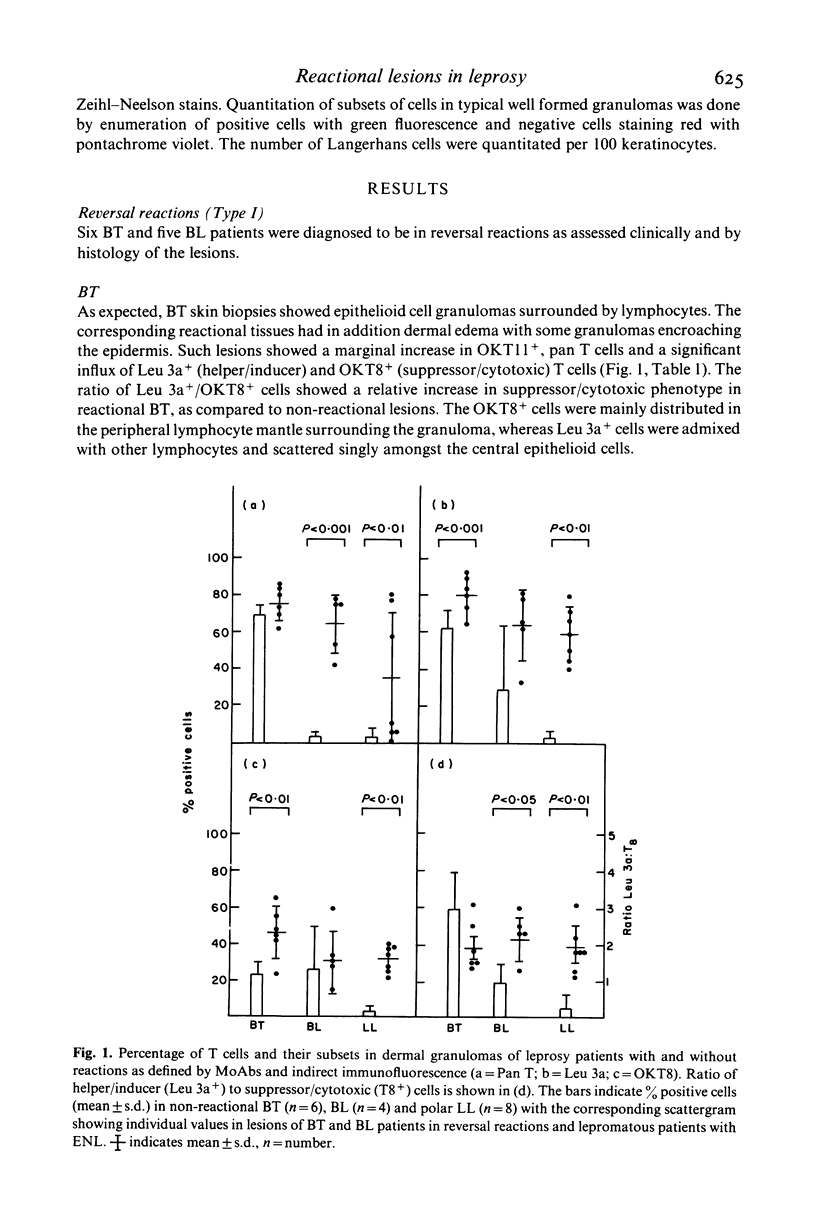
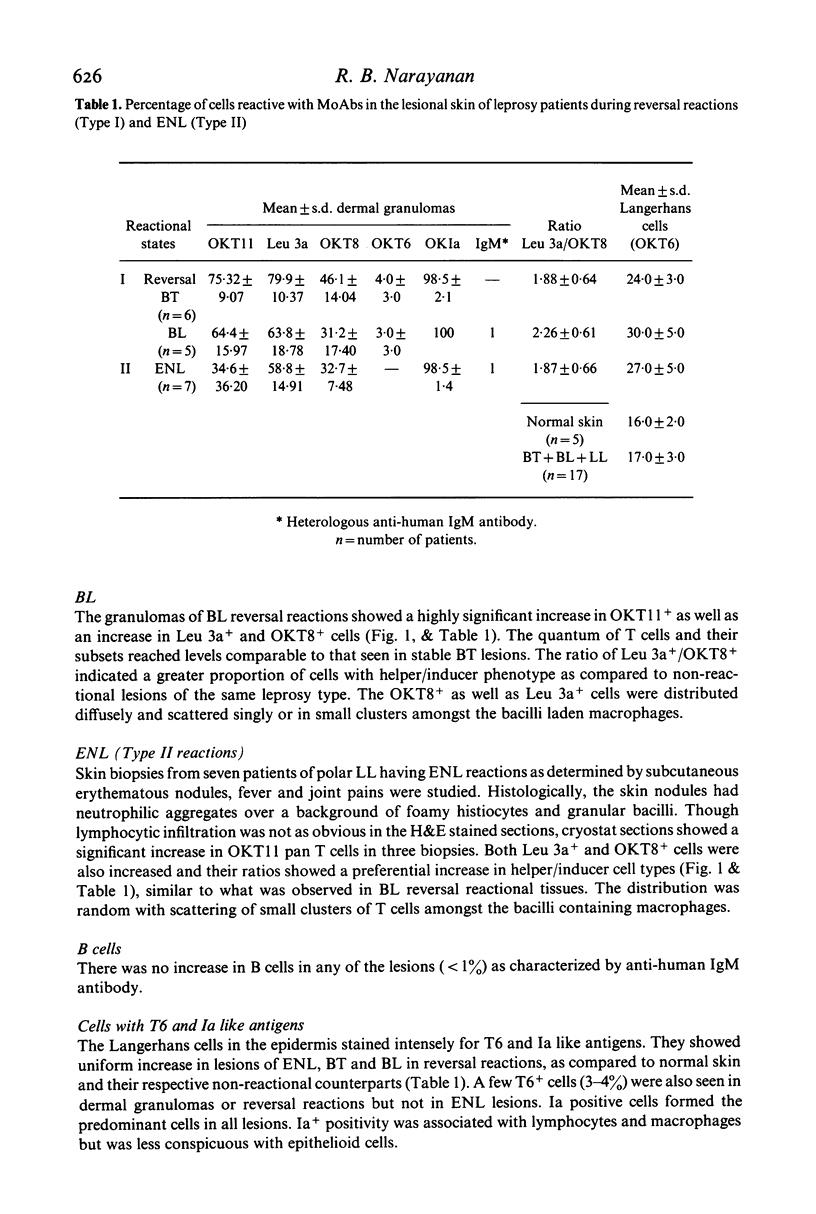


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorvatn B., Barnetson R. S., Kronvall G., Zubler R. H., Lambert P. H. Immune complexes and complement hypercatabolism in patients with leprosy. Clin Exp Immunol. 1976 Dec;26(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Hofman F. M., Meyer P. R., Sharma O. P., Taylor C. R., Rea T. H. In situ demonstration of T lymphocyte subsets in granulomatous inflammation: leprosy, rhinoscleroma and sarcoidosis. Clin Exp Immunol. 1983 Mar;51(3):430–438. [PMC free article] [PubMed] [Google Scholar]
- Narayanan R. B., Bhutani L. K., Sharma A. K., Nath I. T cell subsets in leprosy lesions: in situ characterization using monoclonal antibodies. Clin Exp Immunol. 1983 Mar;51(3):421–429. [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- Wemambu S. N., Turk J. L., Waters M. F., Rees R. J. Erythema nodosum leprosum: a clinical manifestation of the arthus phenomenon. Lancet. 1969 Nov 1;2(7627):933–935. doi: 10.1016/s0140-6736(69)90592-3. [DOI] [PubMed] [Google Scholar]


