Abstract
In all eukaryotes, anaphase is triggered by the activation of a protease called separase. Once activated, separase cleaves a subunit of cohesin, a complex that links replicated chromatids before anaphase. Separase and cohesin are conserved from yeasts to humans. Although the machinery for dissolving sister cohesion is conserved, the regulation of this process appears to be more complex in higher eukaryotes than in yeast. Here we report the cloning of full-length human separase cDNA and the characterization of the encoded protein. Human separase was observed at the poles of the mitotic spindle until anaphase, at which time its association with the mitotic spindle was abruptly lost. The dynamic pattern of localization of human separase during cell cycle progression differs from that of fungal separases. Human separase also appears to undergo an autocatalytic processing on anaphase entry. The processed forms of human separase were isolated and the identity of the cleavage sites was determined by N-terminal sequencing and site-directed mutagenesis. The processed catalytic domain was found to be stably associated with the processed N-terminal fragment. Finally, by depletion of endogenous separase with antisense oligonucleotides, we report direct evidence that separase is required for high-fidelity chromosome separation in human cells.
Separation of sister chromatids at anaphase requires precise coordination between cell cycle signals and the proteins that physically link replicated sisters. Chromatid cohesion is established during S phase and is maintained until onset of anaphase (1). The multisubunit protein complex that holds sisters together is called cohesin (2–7). Scc1 subunit of the cohesin complex undergoes a proteolytic processing at the metaphase-to-anaphase transition, resulting in dissolution of the association between sister chromatids (8). The cleavage of Scc1 appears to be both necessary and sufficient for initiation of anaphase and pole-ward movement of chromosomes (8–10). The specific and highly regulated cleavage of Scc1 subunit is carried out by a cysteine protease termed separase (9, 11, 12). In yeast, the entire pool of cohesin that is bound to sister chromatids (including the chromosome arms and centromeric regions) must be cleaved by separase to initiate anaphase (13). In vertebrates, most of the cohesin dissociates from chromatids in prophase, before chromatid separation in anaphase. A small fraction of cohesin remains in centromeric regions (10, 14). Dissociation of cohesin from prometaphase chromosomes appears to be mediated by a Polo kinase-dependent mechanism (15–17). However, both in yeast and vertebrates, complete cleavage of the chromosome-associated cohesin appears to be an essential prerequisite for initiating anaphase.
Separase belongs to the CD clan of cysteine proteases (8). All members of this class share considerable homology within the domain that contains the active site (18, 19). Another subclass of CD clan endopeptidases are the caspases (20). Caspase activation involves proteolytic processing of the proenzyme form to an activate processed form. Interestingly, what appear to be processed forms of separase were observed after metaphase when human separase is activated (10, 21).
The mechanisms leading to separase activation are a major topic of current research. During the most of the cell cycle separase forms a complex with securin, a protein inhibitor of the separase activity (22–25). Release of the securin-mediated inhibition is achieved by anaphase-promoting complex-dependent ubiquitination and degradation of securin (26, 27). In its turn, activity of the anaphase-promoting complex is controlled by Mad2, a component of the mitotic checkpoint, which ensures that all kinetochores become attached to microtubules (reviewed in ref. 28). The proteolysis of securin is necessary for activation of separase, but securin also appears to have a positive role in facilitating separase activation (6, 29). Separase activity has recently been shown to be inhibited by the cyclin-dependent kinase Cdc2 (21). A third level of regulation occurs by the phosphorylation of the separase substrates. In yeast, phosphorylation of Scc1 near the cleavage site by the Polo-like kinase Cdc5 facilitates separase cleavage (30).
The requirement for separase in dissolving sister cohesion has been directly demonstrated in yeasts by genetic analysis (8, 9, 11, 13, 31). An analogous role for separase in animal cells has been inferred from two lines of evidence. First, vertebrate Scc1 is a substrate of activated vertebrate separase. Second, tissue culture cells overexpressing a noncleavable form of human Scc1p fail to undergo sister chromatid separation (32). The hallmarks of this defect in sister separation are the presence during anaphase of chromosomal bridges, multinucleated cells, and multipolar spindles.
In this study, we directly characterized the role of human separase in mitosis. We find that human separase is associated with centrosomes until anaphase, at which time spindle association is abruptly lost. This event is correlated with the known timing of separase activation and the apparent processing of separase. By protein purification, microsequencing, and site-directed mutagenesis, we identify the sites of human separase cleavage at anaphase. The sequence of the sites coupled with the analysis of a catalytically inactive human separase mutant suggests that separase processing is autocatalytic. Finally, the role of human separase in sister separation was directly tested by depletion of separase with antisense oligonucleotides (ASO). Together, these findings define novel and conserved features of the human enzyme that dissolves sister chromatid cohesion.
Materials and Methods
Separase cDNA.
The 5′-RACE PCR was performed by using Marathon RACE kit, HeLa marathon-ready double-stranded (ds)cDNA (CLONTECH), and separase specific primers 5′-CCTCCAGCATCCAGACAACGGTAGATT-3′ and 5′-GCCAAATCAACTATCTGACAG-3′. Major PCR products ranging from 850 bp to 1.4 kb were cloned into pCR-BluntII-Topo vector (Invitrogen) and sequenced (see also Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org).
Antibodies.
A fragment of human separase corresponding to amino acids 1866–1966 was expressed in Escherichia coli as a GST fusion protein and used for immunization of rabbits (Babco, Richmond, CA). The same separase fragment was also expressed as maltose-binding protein (MBP) fusion protein by using pMal-c2X (New England Biolabs), purified, and coupled to CNBr-activated Sepharose 4B (Apbiotech/Pharmacia). Immune serum was affinity purified on the column with immobilized MBP-separase as described (33). Monoclonal antiseparase antibodies XJ-13 were raised against the same C-terminal separase fragment used to generate the polyclonal antibody, with the exception that an MBP-separase fusion protein was used for immunization of mice instead of the GST-separase fusion.
Cell Lines.
The human cervical carcinoma HeLa cell line was obtained from American Type Culture Collection (ATCC) and was cultured in DMEM (Mediatech, Herndon, VA) supplemented with 10% Fetal Clone-I serum (HyClone), 100 units/ml penicillin, and 100 μg/ml streptomycin. Cell lines expressing either wild type of mutant separase were prepared from 293EcR cell line (Invitrogen). V5-epitope-tagged separase was inserted in pIND-V5–6His-Topo vector (Invitrogen). Transfection of the 293EcR cell line and selection of the positive clones on G-418 (0.4 mg/ml) were carried out as described in the manufacturer's protocol (Invitrogen). The cell lines were induced by Ponasterone (Invitrogen) at a final concentration of 5 μM.
ASO Transfection.
ASO transfection was carried out as described (34) with some modifications. Subconfluent HeLa cells were subjected to mitotic shake-off procedure to enrich in population of the cells in G2/M stage. The cells were allowed to attach for 2 h on six-well plates (Falcon) or glass coverslips and then were transfected with 500 pmols per well of various oligonucleotides and 10 μl per well of Lipofectamine 2000 reagent (Invitrogen). The separase-specific oligonucleotides (synthesized as phosphothiorates to increase stability) are in Fig. 6A, which is published as supporting information on the PNAS web site. FITC-labeled random oligonucleotide was used as a tracer (10 pmols/well), and scrambled oligonucleotide 7261 was used as a control.
Western Blot and Immunostaining.
Western blot was performed according to ref. 33. For immunostaining, cells were cultured and transfected on glass coverslips. Cell staining was carried out as described elsewhere (35).
Results
Autocatalytic Processing of Separase.
The ORF for KIAA0165 ORF predicts a 1,795-aa polypeptide that shares substantial homology with yeast orthologue of separase, Esp1 protein (36). We have identified an additional 5′-exon human separase by 5′-RACE PCR. The full-length human separase cDNA encodes a protein of 2,121 amino acids (see for details, see Supporting Results, which are published as supporting information on the PNAS web site). This cDNA was used to prepare the V5-epitope tagged wild-type separase and other separase mutants used in this study.
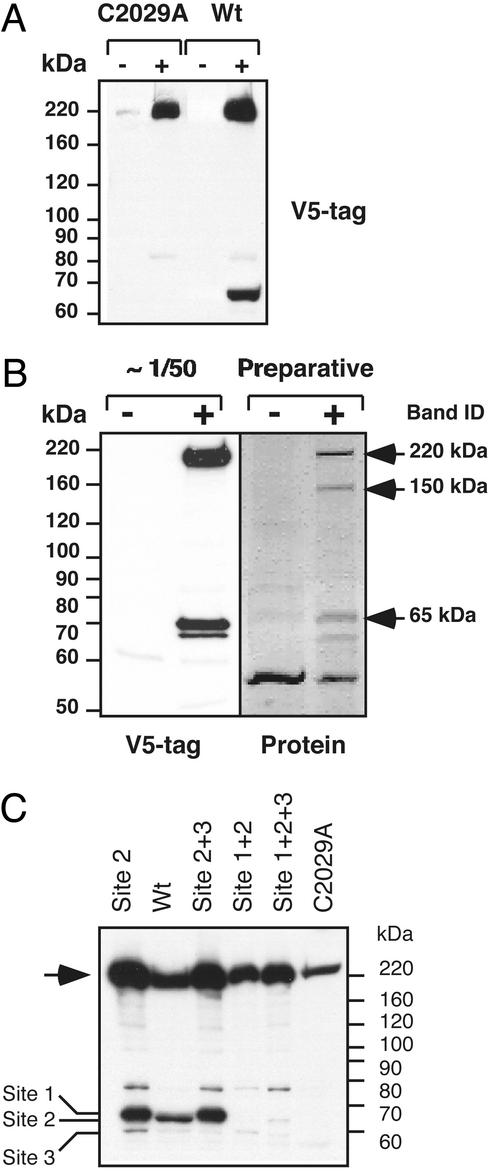
Using the full-length separase cDNA, we characterized the posttranslational processing of human separase. We generated inducible cell lines expressing either V5-tagged wild-type separase or a separase mutant that is predicted to be catalytically inactive, C2029A (8, 32). The cysteine at the position 2029 of separase is essential for proteolytic activity and is conserved among all cysteine proteases (8). The separase cDNA or the C2029A mutant was expressed in 293 EcR cells under the control of the ecdysone promoter that is induced by ponasterone. We found that the wild-type separase expressed 12–48 h postinduction appeared as two polypeptide bands: a full-length protein (220 kDa) and a 65-kDa fragment (Fig. 1A). The 220-kDa (p220) and 65-kDa (p65) polypeptides were present in apparently equal amounts. Both polypeptides were detected by an anti-V5 monoclonal antibody, suggesting that the full-length and truncated forms both contain an intact C terminus. In contrast, induction of the C2029A mutant of separase expression resulted in the appearance of only the full-length molecule; no cleavage products were detected even on prolonged induction up to 72 h (data not shown). This finding suggests that separase processing may be autocatalytic because prolonged incubation of the C2029A separase in the presence of functional endogenous separase did not result in cleavage of C2029A separase.
Figure 1.
Autocatalytic activity of separase at multiple sites is required for posttranslational processing of the enzyme. (A) Addition of ponasterone-induced (+) expression of V5-epitope C-terminally tagged C2029A mutant separase or wild-type (wt) separase in 293 cells. Uninduced cells (−) were used as a control. The C2029A mutant appears as a single polypeptide band corresponding to the full-length molecule. Expression of the wild-type separase results in appearance of a 65-kDa cleavage product. The membrane was probed with V5 antibodies. (B) Identification of the major cleavage site in human separase. Expression of V5-epitope tagged wild-type separase was induced for 14 h and immunoprecipitated with V5-tag antibodies. Ninety-five percent of the precipitated proteins were separated by SDS/PAGE, visualized by Ponceau S staining (Preparative), or used for Western blotting with V5 antibodies. N-terminal sequencing analysis was performed on the 220-, 150-, and 65-kDa protein bands, indicated by the arrows. Sequencing results are given in the text. + and −, induced and noninduced cells, respectively. (C) C-terminally epitope-tagged separase and mutant derivatives were transiently expressed in HeLa cells, and the whole-cell extracts were analyzed by Western blot with V5 antibodies (see text for the description of the mutants). Full-length separase (arrow) and the proteolytic fragments resulting from cleavage at each of the three sites are indicated (left).
Identification of Separase Cleavage Sites.
Next, we used the inducible cell lines to identify the proteolytic sites in human separase. Large-scale immunoprecipitation by anti-V5 antibodies was performed by using extracts prepared from ponasterone-induced cells. Extracts prepared from the uninduced cells were used as a negative control. Most of the immunoprecipitation reaction was used for sequencing analysis, and a small fraction (≈5%) was used for Western blot analysis with anti-V5-tag antibodies. The precipitated material was separated in SDS/PAGE and transferred to a poly(vinylidene difluoride) membrane. Ponceau S staining of the immunoprecipitated proteins revealed several polypeptide bands: a 220-kDa band corresponding to the full-length separase, a 150-kDa band, and the 65-kDa band (Fig. 1B) (designated p220, p150 and p65, respectively). The intensity of the Ponceau S staining of all three bands was similar. Western blot analysis demonstrated that p220 and p65 retained the C-terminal V5-epitope tag, whereas p150 was not detected by anti-V5 antibodies (Fig. 1B).
N-terminal sequencing of both p220 and p150 revealed that these polypeptides start at position 1 of the full-length separase (1MRSFKRVNFGTL12). Identification of the intact N terminus suggests that maturation of separase does not involve removal of any N-terminal amino acids. Because p150 was not detected by anti-V5 antibodies, we concluded that this fragment represents a C-terminally truncated separase. Interestingly, p150 was specifically immunoprecipitated by anti-V5 antibodies, suggesting that p150 forms a complex with V5-epitope-tagged forms of separase that contain the catalytic domain.
The N-terminal sequence of the 65-kDa band revealed a full match with the internal separase sequence starting from 1507G (1507GSDGEDSASGXK1518). Importantly, the separase sequence preceding the cleavage site matches the consensus sequence that has been deduced from the analysis of other separase substrates, such as human Scc1 subunit of cohesin and yeast Slk19 kinetochore-associated protein (SXEXXR↓) (31, 37).
Separase Contains Multiple Autocleavage Sites.
In cohesin, the arginine residue N-terminal to the separase cleavage site is essential for cleavage (8, 32). Substitution of glutamic acid with arginine in the separase consensus sequence in human cohesin (for example EXXR to RXXE) blocks separase-mediated proteolysis at this site (32). A separase mutant encoding the amino acid substitutions E1503R and R1506E was generated (hereafter referred to as Site 2 mutant for the reasons described below). Transient expression of the Site 2 mutant in HeLa cells revealed that, like wild-type separase, it underwent autoproteolytic cleavage, suggesting the presence of additional autocleavage sites. The slower electrophoretic mobility of the fragment resulting from cleavage of Site 2 mutant relative to the wild type suggested an alternative site upstream of the 1506R↓G1507 site (Fig. 1C). Analysis of the separase sequence in the vicinity of the 1506R↓G1507 revealed two additional potential cleavage sites: 1481GPEIMR↓, and 1530EWELLR↓. To determine whether these were genuine separase cleavage sites, these sites were mutated by double-point substitutions E1483R and R1486E (Site 1) and E1532R and R1535E (Site 3). In addition to the mutations of the single sites, mutants altered at either two or all potential cleavage sites were generated. Western blot analysis of HeLa cells expressing the Site 1 + 2 + 3 mutant failed to detect any specific cleavage products (Fig. 1C). This suggests that any of these sites can be cleaved in vivo. For example, the Site 2 + 3 mutant was cleaved at the Site 1, whereas Site 1 + 2 was cleaved at Site 3 (although at apparently lower efficiency than at the Sites 1 and 3; Fig. 1C). Quantitative Western blotting of the three cleavage products (data not shown) suggests that the major cleavage site was Site 2 (1501SFEILR↓); however, if this site is lost, then cleavage at the other two sites becomes apparent. Taken together, these data suggest that human separase, unlike yeast separase (29) undergoes autocatalytic processing. Further, our analysis of this process suggests that the full-length ORF, encompassing the new 5′-end exon reported here, is functional.
Separase Is Localized in Centrosomes Before Anaphase.
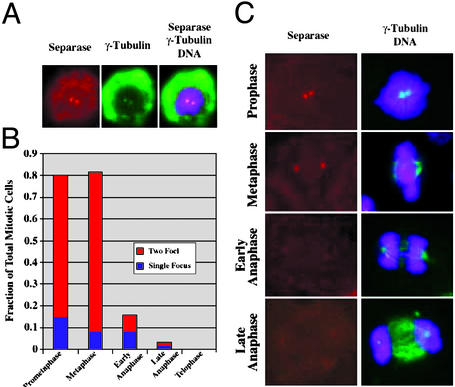
Separase family members Saccharomyces cerevisiae Esp1 and Schizosaccharomyces pombe Cut1 display spindle-pole localization during mitosis. To study the subcellular localization of human separase, polyclonal antibodies raised against the C-terminal 1866–1996 amino acid residue fragment of human separase were used for indirect immunofluorescence. By Western blotting, our affinity purified antibodies detected two major bands corresponding to full-length separase and to the processed C-terminal domain (see Fig. 6C). ASO depletion of separase confirmed that these two major bands correspond to the full-length and processed forms of separase (see below and Fig. 6C). Preliminary cell staining experiments suggested that human separase might localize to the centrosome (data not shown). To test this possibility, double labeling was performed by using anti-γ-tubulin antibodies to identify centrosomes. The double-labeling immunofluorescence experiments in HeLa (Fig. 2 A and C), A549 and U-2 OS (data not shown) cells revealed colocalization of separase and γ-tubulin in centrosomes during the early stages of mitosis. In prometaphase and metaphase, the majority of cells (65.2% and 73.7%, respectively, n > 100) displayed two separase foci that were identified as the centrosomes based on colocalization with γ-tubulin (Fig. 2B). Strikingly, as the cells progressed through anaphase, the separase-specific signal was lost (Fig. 2 B and C). Only 10% of the early and late anaphase cells displayed either one or two foci, and no foci were observed in cells in telophase or during cytokinesis (Fig. 2B, n = 100). The loss of separase signal from the centrosomes was correlated with the proposed timing of separase function at anaphase onset (38). We did not detect the redistribution of separase from the centrosomes onto mitotic spindles, as was observed for the yeast separase (8, 38, 39).
Figure 2.
Cellular localization of human separase: polyclonal antibodies. Shown is immunofluorescence of mitotic HeLa cells, where separase-specific fluorescence is red, γ-tubulin is green, and DNA staining is purple. (A) Prophase HeLa cells displays colocalization of separase (red) with γ-tubulin (green), as evident from superimposition of the images (yellow). (B) Ratio of the number of the cells containing separase labeling of centrosome to the total number of cells at the indicated stages of mitosis (n > 100 cells at each cell cycle stage). Note that anaphase cells were scored as positive for separase even if the staining was fainter than that observed for metaphase cells. The numbers therefore likely underestimate the degree to which separase is lost from centrosomes at anaphase. (C) Immunofluorescence images of prophase, metaphase, and anaphase in HeLa cells showing separase, γ-tubulin, and DNA staining. Shown are representative images from the analysis in B.
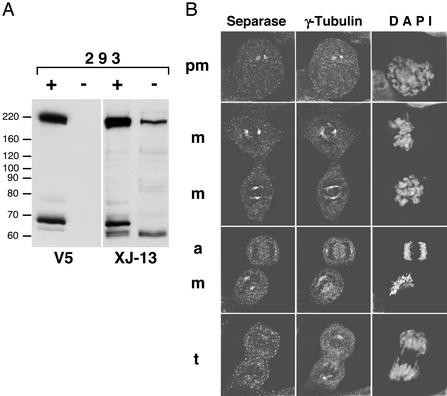
To verify the specificity of the centrosomal localization of human separase, we prepared a monoclonal antibody against the C-terminal fragment of human separase (residues 1866–1996). This mAb (designated XJ-13) recognized both the full-length and autoprocessed forms of recombinant and endogenous separase (Fig. 3B). Of note, by Western blotting, the XJ-13 mAb was highly specific (Fig. 3A). By indirect immunofluorescence, XJ-13 gave the identical pattern of centrosomal labeling as we observed for the affinity-purified polyclonal antibodies. Colocalization of separase with γ-tubulin was observed in 95% of prometaphase cells (n = 110) and in 98% of metaphase cells (n = 120), whereas only 2% of anaphase cells displayed chromosomal localization of separase (n = 105). The centrosome-specific labeling of cells by XJ-13 mAb was blocked by the preincubation of the mAb with the excess antigen (see Fig. 7A, which is published as supporting information on the PNAS web site). Furthermore, the centrosome-specific labeling of cells by XJ-13 was dramatically reduced (from 98% to 30%, n = 200) in prometaphase and metaphase cells transfected with a separase-specific ASO (see below and Fig. 7B).
Figure 3.
Detection of endogenous human separase: monoclonal antibodies. (A) Whole-cell extracts were prepared from control 293 cells (−) or the cells induced to express recombinant V5-tagged full-length separase (+). Identical panels were probed with either V5-tag-specific (V5) or separase-specific (XJ-13) mAbs. (B) Endogenous separase was detected in 293 cells by using XJ-13 mAb and a TRITC-labeled secondary Ab, γ-tubulin was stained by polyclonal Ab, and Cy2-labeled secondary Ab and DNA was visualized by 4′,6-diamidino-2-phenylindole staining. pm, m, a, and t, prometaphase, metaphase, anaphase, and telophase cells, respectively.
Depletion of Endogenous Separase Results in Increase of Aberrant Mitosis.
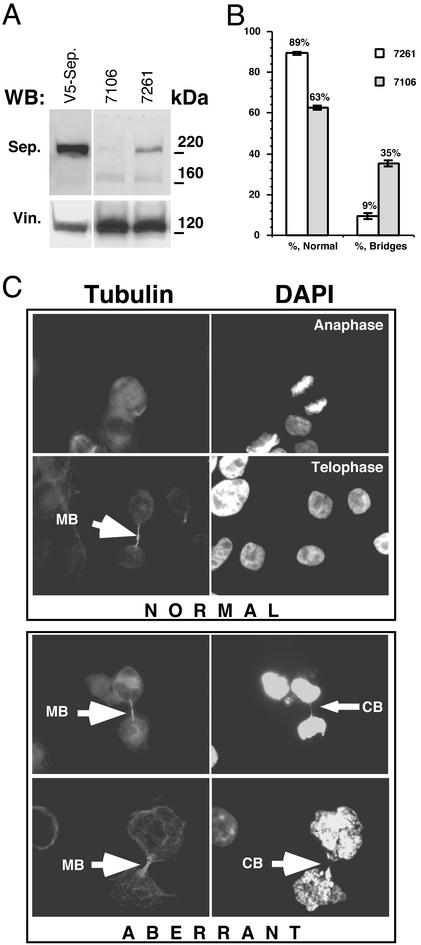
A key role for separase as the trigger for anaphase entry in mammalian cells was suggested by the observation that overexpression of a noncleavable cohesin subunit Scc1 results in abnormal mitosis (32). To directly determine whether separase was required for normal mitosis, we depleted endogenous human separase by use of ASO. A series of various 18-mer oligonucleotides were synthesized and tested for their ability to reduce steady-state level of endogenous separase. HeLa cells were transfected with the indicated ASOs and relative amounts of separase were compared by Western blot by using antiseparase antibodies (Fig. 6B). Although several ASOs resulted in depletion of separase, the most consistent depletion of the endogenous enzyme was achieved with oligonucleotide 7106. To optimize conditions further, the ASO 7106 was tested at various concentrations from 30 to 500 nM. We found that depletion of separase was dosage-dependent and that maximal depletion was achieved at 500 nM. Further increase of the concentration of the ASO 7106 did not result in decrease of the separase signal (data not shown). As a control, a scrambled oligonucleotide with the same nucleotide composition as 7106 was synthesized and designated 7621.
To evaluate the effect of separase depletion on the segregation of sister chromatids, we performed immunostaining of the cells transfected with either 7106 ASO or the control oligonucleotide 7621. Transfection efficiency in the ASO experiments (estimated by cotransfection of an 18-mer fluorescent oligonucleotide) was consistently in the 90–95% range (data not shown). To verify the efficiency of ASO-specific separase knockdown, Western blot analysis of the extracts prepared from transfected cells was performed (Fig. 4A). We found that transfection with 500 nM 7106 ASO resulted in a 10- to 50-fold reduction in separase signal. Immunostaining with antitubulin antibodies was performed to distinguish mitotic and postmitotic cells from total cell population. Fig. 4C (Normal) shows mitotic cells in anaphase that were scored as normal as evident by the absence of lagging chromosomes, whereas cells where lagging chromosomal material was detectable between segregated DNA masses were scored as aberrant (Fig. 4C, Aberrant). The frequency of abnormal mitosis in cells transfected with oligonucleotides 7106 vs. 7621 is shown in Fig. 4B. Antisense oligonucleotide-mediated depletion of separase results in a >4-fold increase in the mitotic cells with lagging chromosomes. This finding directly demonstrates that separase is required for normal chromosome segregation.
Figure 4.
Transfection with various antisense oligonucleotides results in depletion of separase. (A) HeLa cells were transfected with 7106 and control 7621 oligonucleotides at a final concentration of 500 nM. Twenty-four hours after transfection, the cells were harvested in sample buffer and separated in SDS/PAGE. The steady-state levels of separase were compared by Western blot by using antiseparase polyclonal antibodies. V5-Sep., the lane where transfected V5-tagged human separase was loaded as a control. After separase detection, the membrane was stripped and reprobed with anti-Vinculin antibodies (Vin.), confirming the equal loading of the samples. (B) The relative numbers of cells undergoing anaphase after transfection with oligonucleotides 7106 or 7621. The ratio of abnormal bridging chromosomes to the total number of mitotic cells was calculated by counting >500 mitotic cells from three independent experiments. (C) Examples of normal and abnormal anaphase figures from the experiment described in B. MB indicates midbody, and CB indicates a chromosomal bridge.
Discussion
The separase system controls the timing of anaphase onset in all eukaryotes (3, 4, 6, 40, 41). Although the components for sister chromatid separation are conserved, there are important species differences that may be responsible for species-specific regulation. In this paper, we characterize human separase. Our findings emphasize the basic conservation of the system but highlight potentially important unique features of human separase.
The localization of human separase has both similarities and differences from the previously reported localization of yeast separases. Both budding and fission yeast separases localize to the centrosome and to the mitotic spindle (8, 38, 39). In yeast, separase localizes to both the centrosome and spindle either before anaphase (fission yeast) or at anaphase onset (budding yeast) and then persists on the spindle until midanaphase. Using affinity purified polyclonal antiseparase Abs and an antiseparase mAb XJ-13, we have demonstrated that human separase localizes to the centrosomes. We have not observed separase staining along spindle microtubules. Unlike yeast separase, human separase was observed on centrosomes/spindle poles only before anaphase and then was abruptly lost on anaphase onset. Although we cannot exclude the possibility of a spindle-associated pool of separase that is below our detection levels, these findings suggest that human separase may not have the crucial role for maintaining anaphase spindle integrity that has been observed for yeast separase (8, 37–39). The localization of human separase to the centrosome is potentially important, because Caenorhabditis elegans separase was recently shown to be required for migration and cortical association of the paternal centrosome after fertilization. This process is crucial for the establishment of normal anterior–posterior polarity of the embryo (42). Moreover, separase is also required in budding yeast for establishing normal spindle position (43).
Our identification of three cleavage sites in human separase has potential implications for the mechanism of separase activation and regulation. Unlike wild-type separase, a catalytically inactive human separase was not processed in cells containing active endogenous separase (Fig. 1A). The simplest interpretation of this result is that the processing of human separase is autocatalytic. It is interesting to note that Drosophila separase appears to be encoded by two separate genes, one encoding a small protein containing the catalytic domain and another that may function analogously to the N terminus of human separase (44). By contrast, budding yeast separase does not appear to be processed. However, the N terminus of budding yeast separase was recently shown to interact with the C-terminal catalytic domain, explaining why the extreme N terminus is absolutely required separase activity (29). Here we demonstrate that the N-terminal 150-kDa fragment of human separase is tightly associated with the processed catalytic domain. Thus, despite some species differences, an interaction between the N- and C-terminal separase domains may be a common theme for all separases. Although we think it is likely that separase processing follows the initial activation of separase, processing may be important to stabilize the catalytically active conformation or for regulation.
While this manuscript was under review, two studies were published that independently demonstrated the autocatalytic processing of human separase and the physical association of the N- and C-terminal separase fragments (45, 46). Waizenegger et al. (45) identified potential separase autocleavage sites based on the known consensus sequence and by transient expression of potential cleavage site mutants in HeLa cells. Zou et al. (46) identified the same sites by peptide sequencing of processed separase transiently expressed in 293T cells. Both groups found that a separase mutant lacking all three cleavage sites was not detectably compromised for proteolytic activity against the Scc1 subunit of cohesin (45, 46). Additionally, Zou et al. (46) found that overexpressed functional separase can induce the processing of catalytically inactive separase, suggesting that at least some separase processing can occur by an intermolecular reaction. We did not detect processing of catalytically inactive separase in the presence of functional endogenous separase. The intermolecular reaction may therefore be inefficient, raising the possibility that a significant amount of separase processing could occur by an intramolecular reaction.
Our depletion of human separase with antisense oligonucleotides provides direct evidence for a crucial role for separase in separating sister chromatids in human cells. The possibility that the mechanism of sister chromatid separation might differ in some respects between yeast and mammalian cells was raised by the finding that the bulk of mammalian cohesin dissociates from chromosomes well before sister separation at anaphase onset (10, 14). Previous work has established that human separase can cleave the Scc1 subunit of human cohesin (10). Furthermore, noncleavable mammalian Scc1 interferes with sister separation (32). Although these experiments supported the idea that mammalian separase has essentially the same role in separating sisters as yeast separase, an alternate interpretation of these results was that the defect in sister separation was due to an indirect consequence of overexpressing the noncleavable variant of Scc1 rather than a direct effect of failing to cleave cohesin. Here we observe a virtually identical effect of separase depletion on sister separation as that reported for cells expressing noncleavable Scc1 (32), strongly supporting the model that separase activation at anaphase in mammalian cells is the primary trigger for sister chromatid separation.
Supplementary Material
Acknowledgments
We thank Larisa Litovchick for fruitful discussions and help in preparation of the manuscript. This research was supported in part by National Research Service Award 1F32 CA84752 and a research grant to A.C. from the Elsa U. Pardee Foundation (Midland, MI). J.A.D. is a Scholar of the Leukemia and Lymphoma Society, supported by National Institutes of Health Grant RO1-CA63113. D.P. is a Scholar of the Leukemia and Lymphoma Society and is supported by the National Institutes of Health Grant RO1 CA55772.
Abbreviation
- ASO
antisense oligonucleotide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Uhlmann F, Nasmyth K. Curr Biol. 1998;8:1095–1101. doi: 10.1016/s0960-9822(98)70463-4. [DOI] [PubMed] [Google Scholar]
- 2.Orr-Weaver T L. Cell. 1999;99:1–4. doi: 10.1016/s0092-8674(00)80055-0. [DOI] [PubMed] [Google Scholar]
- 3.Nasmyth K. Trends Biochem Sci. 1999;24:98–104. [Google Scholar]
- 4.Amon A. Nat Cell Biol. 2001;3:E12–E14. doi: 10.1038/35050642. [DOI] [PubMed] [Google Scholar]
- 5.Nasmyth K, Peters J M, Uhlmann F. Science. 2000;288:1379–1385. doi: 10.1126/science.288.5470.1379. [DOI] [PubMed] [Google Scholar]
- 6.Yanagida M. Genes Cells. 2000;5:1–8. doi: 10.1046/j.1365-2443.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- 7.Warren W D, Steffensen S, Lin E, Coelho P, Loupart M, Cobbe N, Lee J Y, McKay M J, Orr-Weaver T, Heck M M, Sunkel C E. Curr Biol. 2000;10:1463–1466. doi: 10.1016/s0960-9822(00)00806-x. [DOI] [PubMed] [Google Scholar]
- 8.Uhlmann F, Wernic D, Poupart M A, Koonin E V, Nasmyth K. Cell. 2000;103:375–386. doi: 10.1016/s0092-8674(00)00130-6. [DOI] [PubMed] [Google Scholar]
- 9.Uhlmann F, Lottspeich F, Nasmyth K. Nature. 1999;400:37–42. doi: 10.1038/21831. [DOI] [PubMed] [Google Scholar]
- 10.Waizenegger I C, Hauf S, Meinke A, Peters J M. Cell. 2000;103:399–410. doi: 10.1016/s0092-8674(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 11.Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K. Cell. 1998;93:1067–1076. doi: 10.1016/s0092-8674(00)81211-8. [DOI] [PubMed] [Google Scholar]
- 12.Michaelis C, Ciosk R, Nasmyth K. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- 13.Tomonaga T, Nagao K, Kawasaki Y, Furuya K, Murakami A, Morishita J, Yuasa T, Sutani T, Kearsey S E, Uhlmann F, et al. Genes Dev. 2000;14:2757–2770. doi: 10.1101/gad.832000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Losada A, Hirano M, Hirano T. Genes Dev. 1998;12:1986–1997. doi: 10.1101/gad.12.13.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross K E, Cohen-Fix O. Trends Cell Biol. 2002;12:1–3. doi: 10.1016/s0962-8924(01)02202-4. [DOI] [PubMed] [Google Scholar]
- 16.Sumara I, Vorlaufer E, Gieffers C, Peters B H, Peters J M. J Cell Biol. 2000;151:749–762. doi: 10.1083/jcb.151.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumara I, Vorlaufer E, Stukenberg P T, Kelm O, Redemann N, Nigg E A, Peters J M. Mol Cell. 2002;9:515–525. doi: 10.1016/s1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- 18.Barrett A J, Rawlings N D. Biol Chem. 2001;382:727–733. doi: 10.1515/BC.2001.088. [DOI] [PubMed] [Google Scholar]
- 19.Goyal L. Cell. 2001;104:805–808. doi: 10.1016/s0092-8674(01)00276-8. [DOI] [PubMed] [Google Scholar]
- 20.Thornberry N A, Lazebnik Y. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 21.Stemmann O, Zou H, Gerber S A, Gygi S P, Kirschner M W. Cell. 2001;107:715–726. doi: 10.1016/s0092-8674(01)00603-1. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto A, Guacci V, Koshland D. J Cell Biol. 1996;133:85–97. doi: 10.1083/jcb.133.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou H, McGarry T J, Bernal T, Kirschner M W. Science. 1999;285:418–422. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]
- 24.Leismann O, Herzig A, Heidmann S, Lehner C F. Genes Dev. 2000;14:2192–2205. doi: 10.1101/gad.176700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen-Fix O, Peters J M, Kirschner M W, Koshland D. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- 26.King R W, Deshaies R J, Peters J M, Kirschner M W. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 27.Jallepalli P V, Waizenegger I C, Bunz F, Langer S, Speicher M R, Peters J M, Kinzler K W, Vogelstein B, Lengauer C. Cell. 2001;105:445–457. doi: 10.1016/s0092-8674(01)00340-3. [DOI] [PubMed] [Google Scholar]
- 28.Shah J V, Cleveland D W. Cell. 2000;103:997–1000. doi: 10.1016/s0092-8674(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 29.Hornig N C D, Knowles P P, McDonald N Q, Uhlmann F. Curr Biol. 2002;12:973–982. doi: 10.1016/s0960-9822(02)00847-3. [DOI] [PubMed] [Google Scholar]
- 30.Alexandru G, Uhlmann F, Mechtler K, Poupart M A, Nasmyth K. Cell. 2001;105:459–472. doi: 10.1016/s0092-8674(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 31.Rao H, Uhlmann F, Nasmyth K, Varshavsky A. Nature. 2001;410:955–959. doi: 10.1038/35073627. [DOI] [PubMed] [Google Scholar]
- 32.Hauf S, Waizenegger I C, Peters J M. Science. 2001;293:1320–1323. doi: 10.1126/science.1061376. [DOI] [PubMed] [Google Scholar]
- 33.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 34.Yao X, Abrieu A, Zheng Y, Sullivan K F, Cleveland D W. Nat Cell Biol. 2000;2:484–491. doi: 10.1038/35019518. [DOI] [PubMed] [Google Scholar]
- 35.Chestukhin A, Litovchick L, Rudich K, DeCaprio J A. Mol Cell Biol. 2002;22:453–468. doi: 10.1128/MCB.22.2.453-468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagase T, Seki N, Ishikawa K, Tanaka A, Nomura N. DNA Res. 1996;3:17–24. doi: 10.1093/dnares/3.1.17. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan M, Lehane C, Uhlmann F. Nat Cell Biol. 2001;3:771–777. doi: 10.1038/ncb0901-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumada K, Nakamura T, Nagao K, Funabiki H, Nakagawa T, Yanagida M. Curr Biol. 1998;8:633–641. doi: 10.1016/s0960-9822(98)70250-7. [DOI] [PubMed] [Google Scholar]
- 39.Jensen S, Segal M, Clarke D J, Reed S I. J Cell Biol. 2001;152:27–40. doi: 10.1083/jcb.152.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hixon M L, Gualberto A. Front Biosci. 2000;5:D50–D57. doi: 10.2741/hixon. [DOI] [PubMed] [Google Scholar]
- 41.Uhlmann F. EMBO Rep. 2001;2:487–492. doi: 10.1093/embo-reports/kve113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rappleye C A, Tagawa A, Lyczak R, Bowerman B, Aroian R V. Dev Cell. 2002;2:195–206. doi: 10.1016/s1534-5807(02)00114-4. [DOI] [PubMed] [Google Scholar]
- 43.McGrew J T, Goetsch L, Byers B, Baum P. Mol Biol Cell. 1992;3:1443–1454. doi: 10.1091/mbc.3.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jager H, Herzig A, Lehner C F, Heidmann S. Genes Dev. 2001;15:2572–2584. doi: 10.1101/gad.207301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waizenegger I, Gimenez-Abian J, Wernic D, Peters J. Curr Biol. 2002;12:1368. doi: 10.1016/s0960-9822(02)01073-4. [DOI] [PubMed] [Google Scholar]
- 46.Zou H, Stemman O, Anderson J, Mann M, Kirschner M. FEBS Lett. 2002;528:246. doi: 10.1016/s0014-5793(02)03238-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.