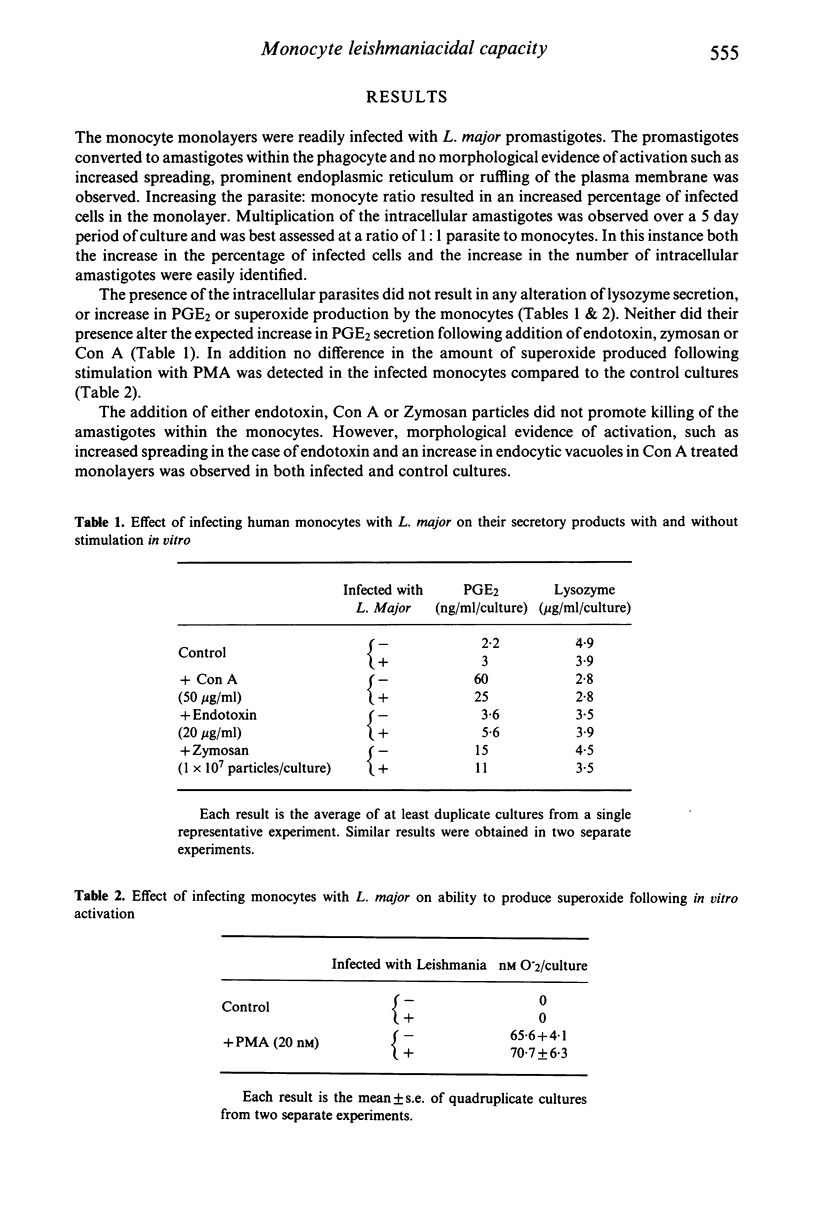
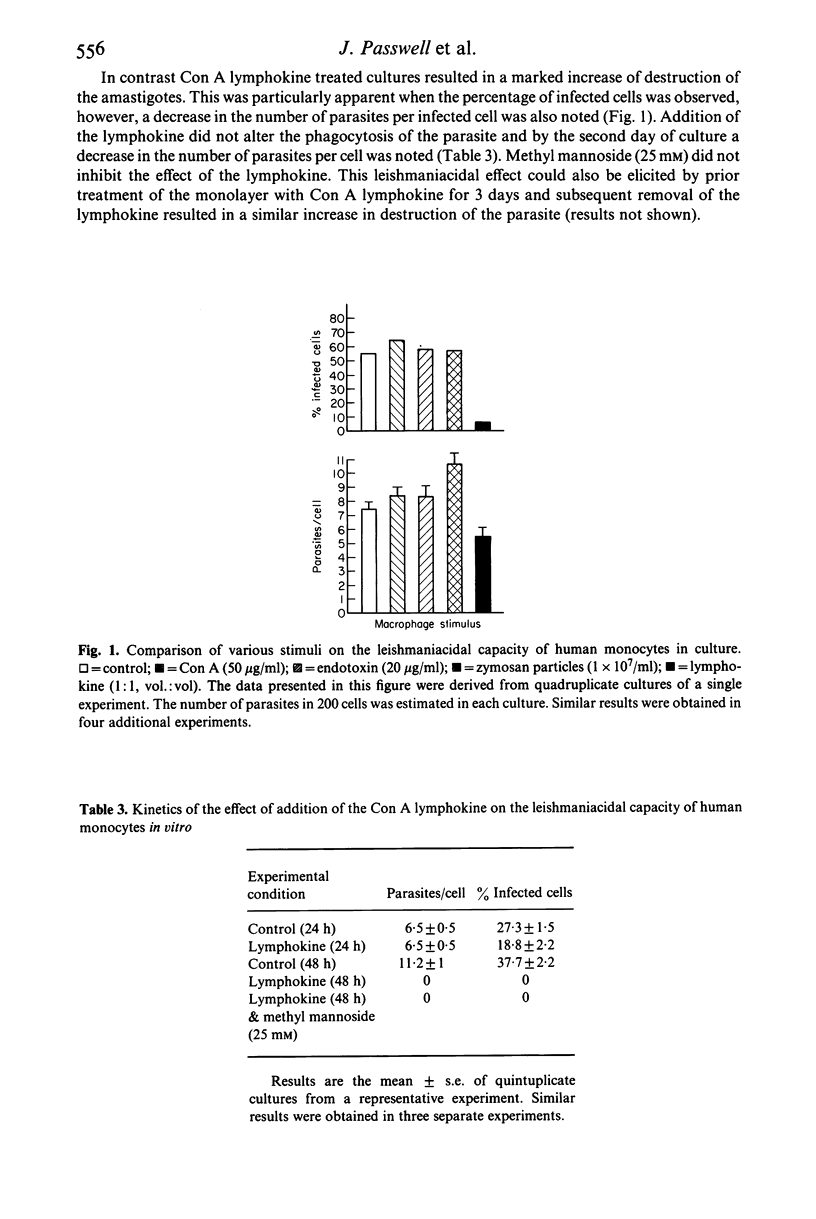
Abstract
Leishmania organisms are obligate intracellular parasites of mammalian mononuclear phagocytes in vivo. In order to study the interactions of these parasites and mononuclear phagocytes, we have used a model of infection of Leishmania major in human monocytes in vitro. The presence of intracellular parasites did not alter the normal secretion of lysozyme or result in increased secretion of prostaglandin E2 (PGE2) or superoxide anion by the monocytes. Addition of concanavalin A (Con A), which binds to a specific membrane receptor, zymosan particles or endotoxin to infected monocyte monolayers, resulted in the expected increase in PGE2 secretion. In addition, the production of superoxide by infected monocytes treated with phorbol myristate acetate was not different from control uninfected cultures. Despite this evidence of biochemical activation, neither endotoxin, zymosan nor Con A had any parasiticidal effect on the intracellular parasites. In contrast, Con A-induced lymphokines from human mononuclear cells resulted in an increased killing of the intracellular amastigotes. These studies have shown that the induction of leishmaniacidal capacity of human monocytes is dependent on the type of stimulus used to induce activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman J. D., Dwyer D. M., Wyler D. J. Multiplication of Leishmania in human macrophages in vitro. Infect Immun. 1979 Oct;26(1):375–379. doi: 10.1128/iai.26.1.375-379.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmüller Y., Mauel J. Studies on the mechanisms of macrophage activation. II. Parasite destruction in macrophages activated by supernates from concanavalin A-stimulated lymphocytes. J Exp Med. 1979 Aug 1;150(2):359–370. doi: 10.1084/jem.150.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. IV. Prophylactic effect of sublethal irradiation as a result of abrogation of suppressor T cell generation in mice genetically susceptible to Leishmania tropica. J Exp Med. 1981 Mar 1;153(3):557–568. doi: 10.1084/jem.153.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine L., Gjtierrez Cernosek R. M., Van Vunakis H. Specificities of prostaglandins B 1 , F 1 , and F 2 antigen-antibody reactions. J Biol Chem. 1971 Nov 25;246(22):6782–6785. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S., Leonard E. J., Wyler D. J. Intracellular replication and lymphokine-induced destruction of Leishmania tropica in C3H/HeN mouse macrophages. J Immunol. 1981 Dec;127(6):2381–2386. [PubMed] [Google Scholar]
- Passwell J. H., Colten H. R., Schneeberger E. L., Marom Z., Merler E. Modulation of human monocyte functions by Fc fragments of IgG: a comparison to other monocyte 'activators'. Immunology. 1980 Sep;41(1):217–225. [PMC free article] [PubMed] [Google Scholar]
- Passwell J. H., Dayer J. M., Gass K., Edelson P. J. Regulation by Fc fragments of the secretion of collagenase, PGE2, and lysozyme by mouse peritoneal macrophages. J Immunol. 1980 Aug;125(2):910–913. [PubMed] [Google Scholar]
- Passwell J. H., Dayer J. M., Merler E. Increased prostaglandin production by human monocytes after membrane receptor activation. J Immunol. 1979 Jul;123(1):115–120. [PubMed] [Google Scholar]
- Passwell J. H., Schneeberger E., Merler E. Cellular requirements for the formation of EA rosettes by human monocytes. Immunology. 1978 Dec;35(6):863–872. [PMC free article] [PubMed] [Google Scholar]
- Petersen E. A., Neva F. A., Oster C. N., Bogaert Diaz H. Specific inhibition of lymphocyte-proliferation responses by adherent suppressor cells in diffuse cutaneous leishmaniasis. N Engl J Med. 1982 Feb 18;306(7):387–392. doi: 10.1056/NEJM198202183060702. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages--induction by multiple nonphagocytic stimuli. Cell Immunol. 1981 Apr;59(2):301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Kaplan G., Sarno E. N., Horwitz M. A., Steinman R. M., Levis W. R., Nogueira N., Hair L. S., Gattass C. R., Arrick B. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N Engl J Med. 1982 Dec 23;307(26):1593–1597. doi: 10.1056/NEJM198212233072601. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. The specificity of suppressor T cells induced by chronic Mycobacterium avium infection in mice. Clin Exp Immunol. 1981 Jan;43(1):10–19. [PMC free article] [PubMed] [Google Scholar]


