Abstract
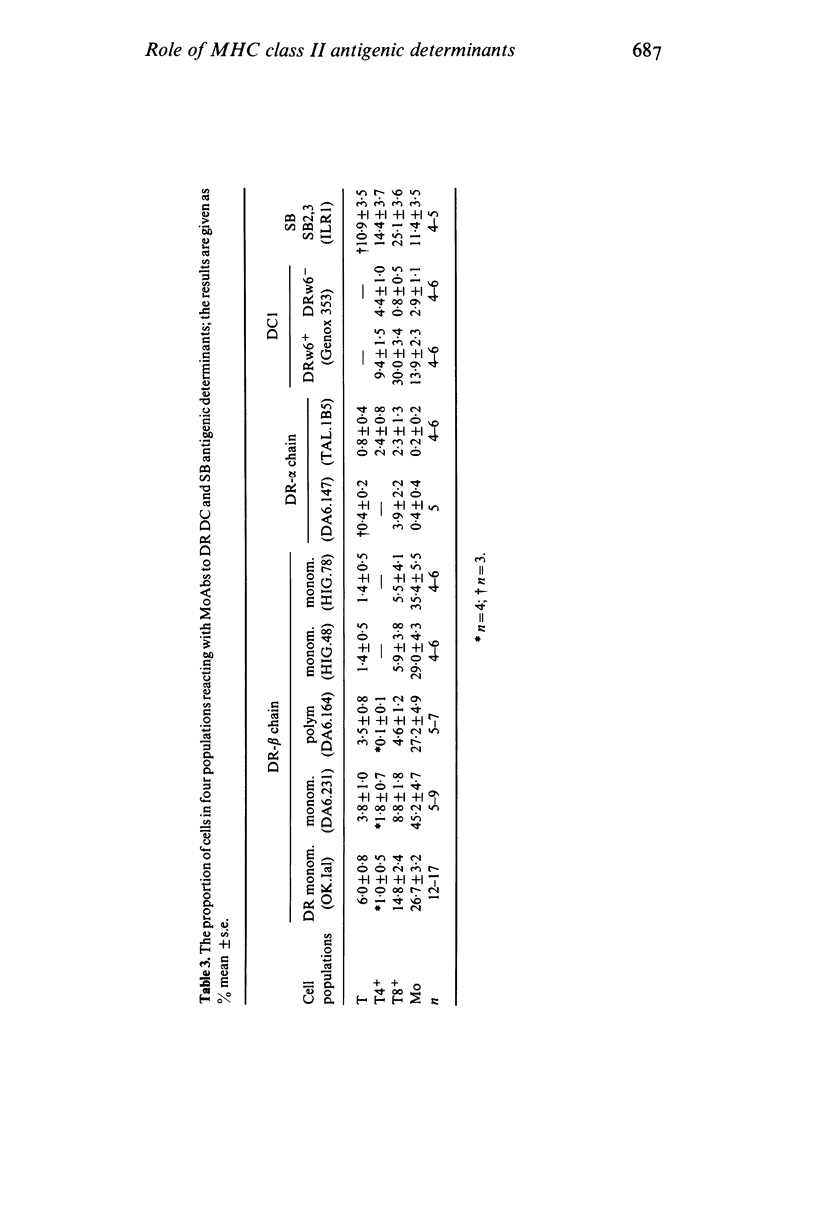
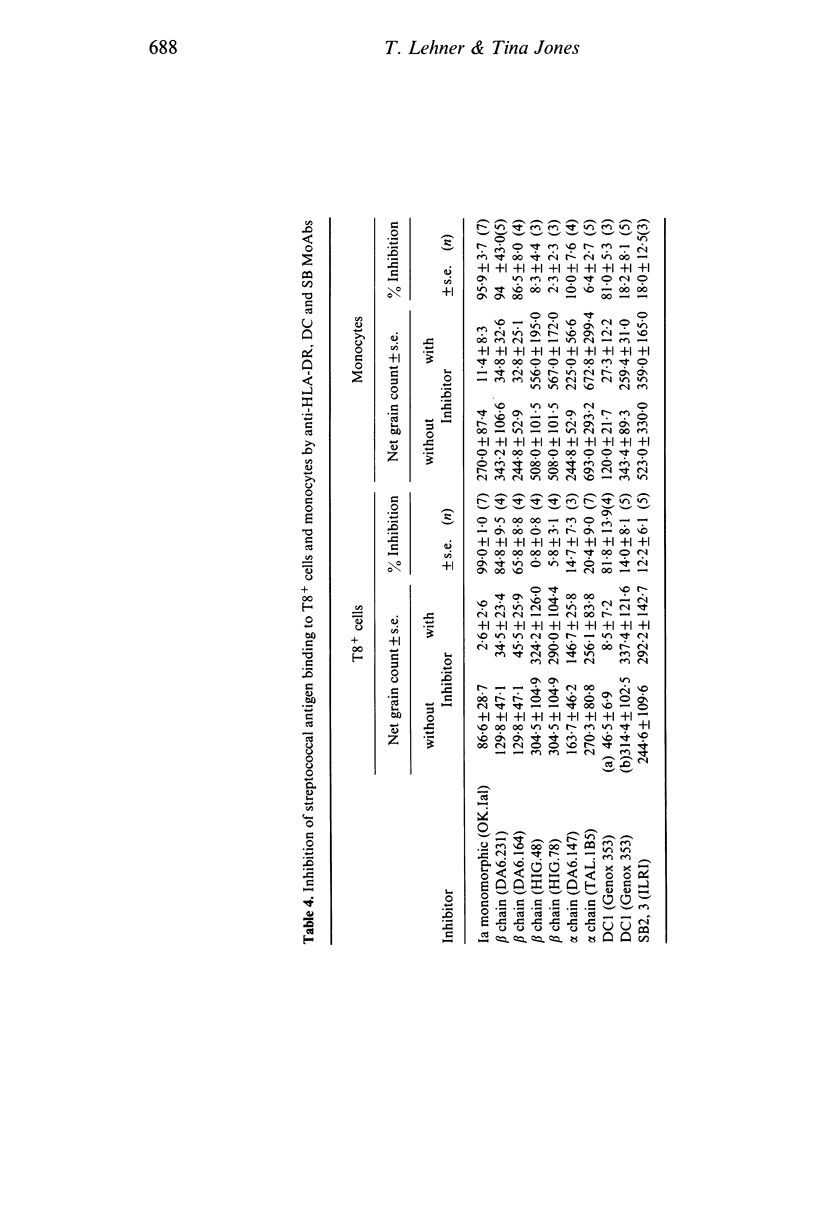
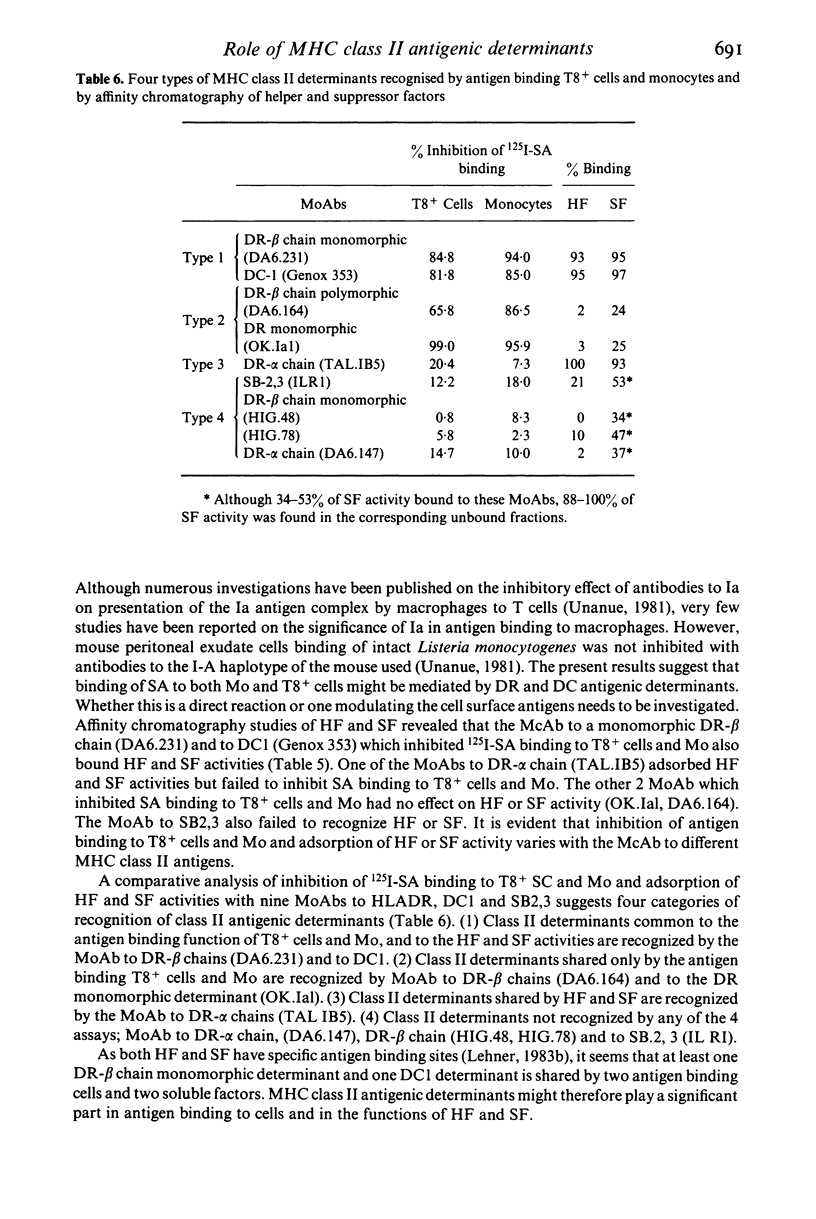
The role of MHC class II antigens was investigated in the process of antigen binding by T8+ cells and monocytes (Mo) and in the functions of helper factor (HF) and suppressor factor (SF). Monoclonal antibodies (MoAbs) to HLA-DR, DC and SB determinants were used in immunofluorescence, inhibition of antigen binding and affinity chromatography of HF and SF. Indirect immunofluorescence studies suggest that T lymphocytes from peripheral blood of healthy subjects have a small proportion of cells expressing HLA-DR, beta chain determinants (1.4-3.8%). These belong predominantly to the T8+ subset of cells (4.6-8.8%), with only a very small proportion in the T4+ cells (0.1-1.8%). However, DC1 on DRw6+ T cells and SB2,3 on any HLA typed cells were found in significantly greater proportion than the DR antigens in both T8+ and T4+ cells, though this was again greater on T8+ (30 and 25%) than T4+ (8.3 and 14.4%) cells. Although Mo had a greatly increased proportion of cells with DR-beta chain determinants (27-45%) than the T8+ cells, the converse was found with DC1 and SB2,3 determinants (13.9 and 11.4%). Inhibition of 125I-streptococcal antigen (SA) binding to T8+ cells and to Mo by MoAbs to the class II antigens showed that DR-beta chain monomorphic or polymorphic antibodies and DC1 antibodies inhibited binding to both cell types by 66-94%. However, MoAbs to DR-alpha chains or to the SB2,3 determinant failed to yield significant inhibition. Affinity chromatography studies of HF and SF revealed that the DR-beta chain monomorphic and DC1 antibodies bound HF and SF activities and that this was not found with the DR-beta chain polymorphic or SB2,3 antibodies. The results of inhibition of 125I-SA binding to T8+ cells and Mo, and absorption of HF and SF by affinity chromatography with MoAbs suggest four categories of recognition of human MHC class II antigenic determinants. (1) Class II determinants shared by the T8+ cells, Mo, HF and SF and recognized by MoAbs to monomorphic beta chains (DA6.231) and to DC1. (2) Class II determinants shared only by the SA binding T8+ cells and Mo and recognized by the MoAbs to a polymorphic beta chain (DA6.164) and to a monomorphic DR determinant (OK.Ial). (3) Class II determinants shared only by the HF and SF and recognized by the MoAbs to one of the alpha chains (TAL.1B5). (4) Class II determinants not detected on the two cells or the two T cell factors.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodmer W. F. HLA structure and function: a contemporary view. Tissue Antigens. 1981 Jan;17(1):9–20. doi: 10.1111/j.1399-0039.1981.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Ceuppens J. L., Goodwin J. S., Searles R. P. The presence of Ia antigen on human peripheral blood T cells and T-cell subsets: analysis with monoclonal antibodies and the fluorescence-activated cell sorter. Cell Immunol. 1981 Nov 1;64(2):277–292. doi: 10.1016/0008-8749(81)90480-9. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Receptors on immunocompetent cells. II. Specificity and nature of receptors on dinitrophenylated guinea pig albumin- 125 I-binding lymphocytes of normal guinea pigs. J Exp Med. 1971 Aug 1;134(2):495–516. doi: 10.1084/jem.134.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Wang C. Y., Montrazeri G., Kunkel H. G., Ko H. S., Gottlieb A. B. Ia-bearing T lymphocytes in man. Their identification and role in the generation of allogeneic helper activity. J Exp Med. 1978 Nov 1;148(5):1423–1428. doi: 10.1084/jem.148.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Verbi W., Festenstein H., Papasteriadis C., Jaraquemada D., Hayward A. "Ia-like" antigens on human T cells. Eur J Immunol. 1979 May;9(5):356–362. doi: 10.1002/eji.1830090504. [DOI] [PubMed] [Google Scholar]
- Guy K., Van Heyningen V., Cohen B. B., Deane D. L., Steel C. M. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur J Immunol. 1982 Nov;12(11):942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Lehner T. Antigen-binding human T suppressor cells and their association with the HLA-DR locus. Eur J Immunol. 1983 May;13(5):370–378. doi: 10.1002/eji.1830130505. [DOI] [PubMed] [Google Scholar]
- Lehner T. Characterization of human helper and suppressor factors with special reference to HLA-DR determinants. Immunology. 1983 Apr;48(4):695–702. [PMC free article] [PubMed] [Google Scholar]
- Lehner T. The relationship between human helper and suppressor factors to a streptococcal protein antigen. J Immunol. 1982 Nov;129(5):1936–1940. [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Sekaly P. R., Moretta A., Chapuis B., Cerottini J. C. Surface markers of cloned human T cells with various cytolytic activities. J Exp Med. 1981 Aug 1;154(2):569–574. doi: 10.1084/jem.154.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurman R. K., Gelfand E. W., Matheson D., Zimmerman B., Dosch H. M. Identification of Ia on a subpopulation of human T lymphocytes that stimulate in a mixed lymphocyte reaction. J Immunol. 1980 Apr;124(4):1924–1928. [PubMed] [Google Scholar]
- Shackelford D. A., Mann D. L., van Rood J. J., Ferrara G. B., Strominger J. L. Human B-cell alloantigens DC1, MT1, and LB12 are identical to each other but distinct from the HLA-DR antigen. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4566–4570. doi: 10.1073/pnas.78.7.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., DeMars R., Schlossman S. F., Smith P. L., Lampson L. A., Nadler L. M. Serologic identification of the human secondary B cell antigens. Correlations between function, genetics, and structure. J Exp Med. 1982 Sep 1;156(3):731–743. doi: 10.1084/jem.156.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Johnson A. H., Shearer G. M. Evidence for a new segregant series of B cell antigens that are encoded in the HLA-D region and that stimulate secondary allogenic proliferative and cytotoxic responses. J Exp Med. 1980 Sep 1;152(3):565–580. doi: 10.1084/jem.152.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snary D., Barnstable C., Bodmer W. F., Goodfellow P., Crumpton M. J. Human Ia antigens--purification and molecular structure. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):379–386. doi: 10.1101/sqb.1977.041.01.045. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Whisler R. L., Wajda K. J., Newhouse Y. G. Phenotypic characteristics of peripheral blood T cells regulating colony growth by nontransformed human B lymphocytes. J Immunol. 1983 Feb;130(2):665–670. [PubMed] [Google Scholar]
- Wilson B. S., Indiveri F., Pellegrino M. A., Ferrone S. Absence of DR antigens on human T lymphoid cells: serologic and immunochemical studies with xenoantisera. Transplant Proc. 1979 Mar;11(1):712–714. [PubMed] [Google Scholar]
- Winchester R. J., Kunkel H. G. The human Ia system. Adv Immunol. 1979;28:221–292. [PubMed] [Google Scholar]
- Yu D. T., McCune J. M., Fu S. M., Winchester R. J., Kunkel H. G. Two types of Ia-positive T cells. Synthesis and exchange of Ia antigens. J Exp Med. 1980 Aug 1;152(2 Pt 2):89s–98s. [PubMed] [Google Scholar]


