Abstract
The link between basal cell carcinoma (BCC) and aberrant activation of the Hedgehog (Hh) signaling pathway has been well established in humans and in mouse models. Here we report the development of assays, including two novel in vitro BCC models, which allowed us to screen for Hh inhibitors and test their validity as potential treatments for BCC. We identified a novel small molecule Hh inhibitor (CUR61414) that can block elevated Hh signaling activity resulting from oncogenic mutations in Patched-1. Moreover, CUR61414 can suppress proliferation and induce apoptosis of basaloid nests in the BCC model systems, whereas having no effect on normal skin cells. These findings directly demonstrate that the use of Hh inhibitors could be a valid therapeutic approach for treating BCC.
Keywords: cancer‖ medulloblastoma ‖patched ‖smoothened
The Hedgehog (Hh) protein family, which includes Sonic (Shh), Indian (Ihh), and Desert (Dhh) hedgehogs, regulates numerous events in embryonic development (1–4). Patched-1 (Ptch-1) serves as the receptor for all of the hedgehog proteins. In the absence of Hh, Ptch-1 inhibits Smoothened (Smo), a G protein coupled-like receptor. When Hh binds to Ptch-1, Smo is disinhibited and initiates a signaling cascade that results in activation of a transcription factor, Gli-1.
In humans, aberrant activation of the Hh pathway is associated with certain cancers, including basal cell carcinoma (BCC) and medulloblastoma (5–8). BCC is the most common cancer in the western world, with well over 750,000 new cases annually in the United States alone (9). Mutations in Ptch-1 (encoded by PTCH1) were first found in the inherited form of the disease known as Basal Cell Nevus Syndrome (BCNS), or Gorlin Syndrome (10), in which patients may develop hundreds of BCCs (5, 6). Patients with BCNS inherit one copy of mutated PTCH1 and develop BCC once the remaining wild-type copy is mutated or deleted in epidermal keratinocytes, presumably after exposure to sunlight (11). Moreover, mutations of components of the Hh signaling pathway, including PTCH1 and SMO, that activate the pathway in the absence of Hh have been found in >70% of sporadic BCCs (8, 12). Importantly, regardless of where the particular oncogenic mutations reside in the pathway, virtually all human BCC samples have elevated levels of a more downstream component of the pathway, the transcription factor Gli-1 (13).
The link between human BCC and Hh signaling has been substantiated in animal studies in which transgenic mice with constitutively activated Hh signaling in the skin developed BCC-like lesions (14–17). Similarly, continuous Hh signaling induced neoplasia by opposing cell cycle arrest, and thus differentiation, of the suprabasal keratinocytes in human skin transplants (18). Forced expression of Shh in the basal keratinocytes prevented p21CIP1/WAF1-induced growth arrest, extended the S and G2/M phases of their cell cycle, and prolonged their replicative capacity (18).
All of this information suggests that compounds that are able to suppress aberrantly activated Hh signaling may cause growth arrest of BCC cells and may provide an effective chemotherapeutic for BCC. Previously, a cell-based system had been used to show that the plant-derived Hh inhibitor cyclopamine can block the oncogenic activation of the Hh signaling pathway and decrease proliferation of cultured mouse fibroblasts (e.g., NIH 3T3 cells) (19). However, these studies were limited, being based on the action of inhibitors on cell lines. The effect of antagonizing Hh signaling on human BCC lesions has not been reported, largely because of the difficulty in culturing BCC-derived materials.
Here we report several assay systems for the identification of synthetic small molecule inhibitors of the Hh signaling pathway and validation of the effectiveness of these inhibitors in the treatment of BCC-like lesions. To achieve the latter, two novel in vitro BCC culture systems have been developed: one in which mouse embryonic skin punches were treated with a highly active form of Hh protein to induce basaloid nests (reminiscent of basal islands in BCC) and another in which adult mouse skin explants containing BCC-like lesions, derived from Ptch+/− heterozygous mice after long-term UV irradiation, were maintained in culture. A potent small molecule Hh inhibitor (CUR61414) arrested proliferation of basal cells within the BCC-like lesions and induced them to undergo apoptosis leading to complete regression of the lesions, without affecting neighboring skin cells. Combined with recent results on the effects of cyclopamine on medulloblastoma growth (20), our data suggest that inhibitors of the Hh pathway may be effective chemotherapeutics to treat cancers that arise from mutations in that pathway.
Materials and Methods
Screening for Hh Inhibitors.
C3H.10T1/2 cells (American Type Culture Collection) were grown in DMEM/10% FBS, with penicillin/streptomycin/glutamine. The Hh-responsive (Gli8x-luciferase) reporter construct (a gift from Brigid Hogan, Vanderbilt University, Nashville, TN) was transfected into the 10T1/2 cells, and stable clones were selected in 1 μg/ml Geneticin (GIBCO/BRL, Grand Island, NY). In these studies, a modified form of Shh that has an octyl moiety coupled to its N terminus (ShhOCT) was used; this lipid-modified protein has shown to be much more potent in cell-based assays (21). The clone (s12) showing the strongest induction of luciferase activity (8- to 10-fold) on addition of ShhOCT was selected for use. To identify novel inhibitors, various collections of small molecules were screened in this assay. For this purpose, s12 cells were plated in 96-well plates at 20,000–30,000 cells per well and grown for 24 h before compound addition, in DMEM/10% FBS (with penicillin/streptomycin/glutamine). Next, the plates were changed to medium with 0.5% FBS, and compounds (2–5 μM each) were added in the presence of 0.5 μg/ml ShhOCT. After 24 h, plates were assayed for luciferase activity with the LucLite kit (Packard, Meriden, CT). To ensure specificity for the Hh signaling pathway, compounds were subjected to a simian virus 40 (SV40)-luciferase counterscreen in which the luciferase activity was constitutively present, independent of Hh signaling (data not shown). Only those compounds that showed inhibition of the Hh-induced, and not the SV40-dependent, luciferase activity were further tested.
Chick Explant Assay.
Intermediate (“naïve”) regions of embryonic chick neural tube (stage 10–11) were dissected, embedded in collagen, and cultured as described before (22–24). These explants (n = 6) were then treated with vehicle alone, ShhOCT, or ShhOCT + test compound. After 22 h, explants were fixed and stained with antibodies against Pax7 and Nkx2.2.
Ptch-Null Assay.
To generate a “Ptch-Null” cell line, several Ptch−/− (Ptch-Null) mouse embryonic stem (ES) cell clones were isolated from a parental Ptch+/− (Ptch-heterozygote) ES cell line in the presence of G418 (1 mg/ml). One of the clones was injected into nude mice to generate teratomas. Teratomas were harvested 4 weeks later and dissociated into single cell suspensions for culturing. A spontaneously immortalized and fibroblast-like (Ptch-Null) cell line, Tera-28, was established from the culture. For compound testing, Tera-28 cells were grown in DMEM with 10% FBS and incubated with the compound (1–5 μM) for 72 h. Total RNA was then isolated from the cells (RNA Isolation kit from Qiagen, Valencia, CA) for detecting Gli-1, β-actin, and GAPDH transcripts by conventional RT-PCR and TaqMan analysis (Applied Biosystems, Foster City, CA). The β-actin (for gel analysis) and GAPDH (for TaqMan) transcripts were used for normalizing PCR results for Gli-1. PCR products from conventional RT-PCR were analyzed by gel electrophoresis on 1.5% agarose gels.
Embryonic Skin Punch Assay.
Mouse embryos, from Ptch+/−-LacZ mice, were collected and killed at late gestation (embryonic day 17.5) and their skins excised. Circular punches (4 mm in diameter) were placed in a collagen-coated Transwell (BIOCOAT Cell Culture Insert, Becton Dickinson Labware, Bedford, MA) and cultured at the air–liquid interface, with the epidermis side facing up. The culture medium contained 5% FBS in DMEM/F12 (3:1) with added epidermal growth factor, insulin, and hydrocortisone. To induce formation of basaloid nests, punches were grown in the presence of 1–2 μg/ml ShhOCT for 4 or more days. Effects of antagonists were tested by adding compounds at the time of Shh addition or after 6 days of Shh pretreatment.
Adult Skin Punch Assay.
Ptch+/−-LacZ heterozygous mice were crossed to hairless mice (hr/hr) to generate [(Ptch+/−)(hr/hr)] double mutants. The mice were irradiated with a Daavlin UV-B research irradiation unit with UV-B integrating dosimetry (Daavlin, Bryan, OH), at 6.75 mJ/cm2, three times per week for 6–9 months, much as described by Aszterbaum et al. (15). After irradiation, skin biopsies were taken from each mouse and inspected for BCC lesions. Skin punches from UV-irradiated mice were cultured and treated with Hh inhibitor as described for the embryonic skin punch assay.
5-Bromo-4-chloro-3-indolyl β-d-Galactoside (X-gal) Staining.
For X-gal staining, skin punches were fixed for 30 min at room temperature (RT) and washed with PBS. The samples were then placed in staining buffer containing 1 mg/ml of X-gal (Sigma), and incubated overnight at 37°C. Subsequently, samples were rinsed with PBS before fixation in 4% paraformaldehyde for 24 h. Fixed punches were processed for histological analysis.
Histological Analysis, Immunocytochemistry, and Apoptosis Assays.
Skin punches were first fixed in 4% paraformaldehyde for 24 h and then processed for paraffin embedding and sectioning. For general histological analysis and counter staining, sections were stained with hematoxylin and eosin (H&E) or light eosin alone (Vector, Burlingame, CA). For immunohistochemistry, the VECTASTAIN Elite kit (Vector Laboratories) was used. Slides were incubated with antibodies for 45 min at RT, at the following dilutions: α-CK14 (cytokeratin 14), 1:40; α-CK10, 1:10; α-Loricrin, 1:60; α-PCNA (proliferating cell nuclear antigen), 1:50. Cell death was assessed with the DeadEnd Colorimetric Apoptosis Assay kit (Promega).
Effect of CUR61414 on Proliferation and Apoptosis.
Eight or more skin punches (4 mm) were prepared from each of 15 embryonic mice and cultured as described. After 6 days of incubation with ShhOCT protein to allow the formation of basaloid lesions, four were further treated with vehicle (0.5% DMSO) and sets of four were treated with different concentrations of CUR61414 in vehicle. One of the four punches from each set was then processed for detailed analysis of cell proliferation and apoptosis. For this purpose, punches were fixed, embedded and sectioned. The sections were then stained with an anti-PCNA antibody to measure the amount of proliferation, and the terminal deoxynucleotidyltransferase-mediated dUTP end labeling (TUNEL) assay was used on adjacent sections to quantify the degree of apoptosis. Each section contained ≈8–14 individual lesions. Lesions toward the edges of the sections were excluded from all calculations. For each lesion, the total number of cells was measured, as was the number of either PCNA+ (proliferating) or TUNEL+ (apoptotic) cells. Mitotic and apoptotic indices were then calculated as percent of total cells and averaged across the 15 individual punches for each condition.
Effect of CUR61414 on the UV-Induced Lesions.
Skin punches (4 mm) were generated from UV-irradiated Ptc+/−-lacZ animals and cultured. After treatment with vehicle or CUR61414, sections were fixed and stained with X-gal. The number of basaloid lesions (recognized by their blue color) in each punch, regardless of size, was then counted by a blinded observer. Histological analysis showed that individual lesions contained between ≈50 and 300 cells (data not shown). Three experiments, each containing four separate skin punches, were analyzed.
Supporting Information.
Additional materials and methods are described in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org.
Results
Identification of a Small Molecule Hh Inhibitor.
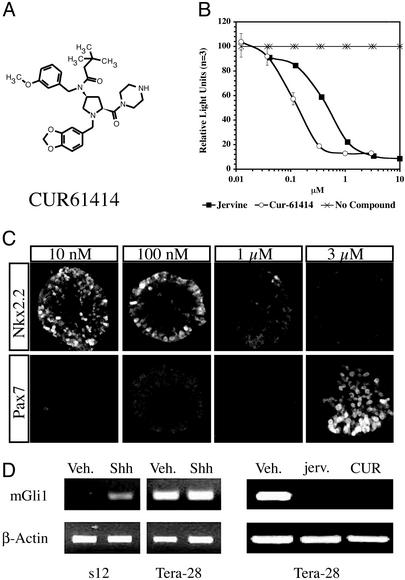
To screen for small molecule inhibitors of the Hh signaling pathway, an Hh-responsive reporter cell line (s12) was developed and then used in a high-throughput screen against a library containing ≈100,000 small synthetic organic molecules. One of the lead compounds identified was an aminoproline, CUR61414 (Mr 513 Da; Fig. 1A). CUR61414 is a significantly more potent inhibitor (IC50 = 100–200 nM, Fig. 1B) of Hh-induced activity than is the previously identified natural product Hh inhibitor jervine (25), which showed an IC50 of 500–700 nM under our experimental conditions (Fig. 1B).
Figure 1.
Identification of a small molecule Hh inhibitor. (A) Schematic representation of the chemical structure of CUR61414. (B) Inhibitory activity of CUR61414 in the Hh-reporter assay; comparison with jervine. (C) CUR61414 reverses Hh-induced expression of neural markers (Pax7, Nkx2.2) in the chick neural plate explant assay. (D Left) Response of the Hh reporter cells (s12) and Ptch-Null cells (Tera-28) to ShhOCT, at 0.5 μg/ml (Veh., vehicle; Shh, ShhOCT). (D Right) Expression of Gli-1 message in Ptc-Null (Tera-28) cells, in the presence of vehicle (Veh., 0.5% DMSO), jervine (jerv., 5 μM), and CUR61414 (CUR, 1 μM) analyzed by RT-PCR (β-actin is the internal control).
The ability of CUR61414 to act as a true hedgehog inhibitor was confirmed by using a chick neural plate explant assay (22, 23). Neural progenitors in the developing chick neural tube, as they differentiate in response to a gradient of Hh protein, undergo a specific sequence of expression of the transcription factors Pax7 and Nkx2.2. Pax7 increases at very low Hh concentrations, but, as the concentration of Hh is elevated, Pax7 is then down-regulated, whereas Nkx2.2 is up-regulated (23, 26, 27). The expression patterns of these markers were examined in neural tube explants treated with Hh protein and with various concentrations of CUR61414. Although it was slightly less potent on chicken than on mammalian cells, CUR61414 was able to suppress changes in both markers in a dose-dependent fashion (Fig. 1C). Interestingly, the pattern of suppression appeared to be the inverse of the pattern of induction: Nkx2.2 decreased at higher inhibitor concentrations, whereas Pax7 first increased then decreased as Hh activity was progressively inhibited (Fig. 1C). These results confirm that CUR61414 can inhibit Hh signaling in a well established Hh assay. We also determined that CUR61414 did not block other developmentally regulated signaling pathways, like those for BMP and Wnt (see Figs. 5 and 6, which are published as supporting information on the PNAS web site).
Recently published work (23, 28, 29) has demonstrated that CUR61414, jervine, and certain other classes of small molecule Hh antagonists bind directly to Smo, a G protein-coupled receptor. Therefore, it was also important to show that CUR61414 does not have significant inhibitory effects on other G protein-coupled receptors. This was established in a series of radioligand binding assays in which the Hh inhibitor was shown not to affect ligand binding for a variety of other receptors of this class (Table 3, which is published as supporting information on the PNAS web site). In sum, this work demonstrates that CUR61414 is a specific Hh pathway inhibitor.
CUR61414 Blocks Hh Signaling in Ptch-Null Cells.
Because most mutations associated with the Hh signaling pathway in human BCC lesions arise in PTCH1, we decided to test the efficacy of CUR61414 on cell lines carrying inactive Ptch-1. As expected, this compound, which acts downstream of Ptch-1, strongly inhibited Hh signaling in these cells (Fig. 1D). Similar results were observed with quantitative RT-PCR (TaqMan) analysis (Fig. 7, which is published as supporting information on the PNAS web site).
An in Vitro BCC Model System.
Having shown that CUR61414 can prevent the activation of the Hh signaling pathway in cells that have a mutation commonly observed in BCC, we wanted to test its activity of these inhibitors in the context of normal skin and BCC-like skin lesions. For this, we set out to develop an in vitro BCC model system.
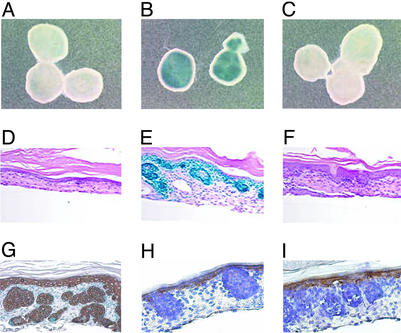
It had been shown previously that basaloid nests develop in CK14-Shh transgenic mice and in human skin in which Shh is overproduced in basal keratinocytes (14, 30). Building on these observations, we established a skin culture system in which punches from mouse embryonic skin, derived from Ptch+/−-LacZ heterozygote mice, were maintained in vitro in the presence of recombinant Shh protein. Because PTCH1 is a target of Hh signaling, the Ptch-lacZ transgene allowed us to monitor Hh signaling by following β-galactosidase activity. Shh addition to the embryonic skin caused strong activation of its signaling pathway (Fig. 2 A and B). Histological analysis of the Hh-treated skin showed large nodular clusters of basal cells (basaloid nests), absent in vehicle treated samples, and reminiscent of the basal cell islands observed in BCC (Fig. 2 D and E). These nodular clusters appeared as soon as 4 days after protein treatment (data not shown), but only developed fully after 6 days. Intense X-gal stain, reflecting elevated Hh signaling, appeared restricted to these nodular clusters (Fig. 2E), except for staining associated with some basal keratinocytes and hair follicles, also known to be sites of Hh signaling.
Figure 2.
Skin punches from embryonic day 17.5 Ptc+/−-lacZ embryos form BCC-like structures in response to treatment with Hh. (A–C) Whole-mount X-gal staining of intact punches after treatment with no ShhOCT added (A), 1 μg/ml ShhOCT (B), or 1 μg/ml ShhOCT + 1 μM CUR61414 (C). (D–F) H&E counterstained sections from the cultured skin punches in A–C, respectively. (G–I) Expression of typical epidermal markers in embryonic skin after treatment with Hh, for 6 days: α-CK14 (G), α-CK10 (H), and α-loricrin (I). Note that the basal keratinocyte marker, CK14, is highly expressed in the basaloid lesions (G).
The Hh-induced basaloid nests showed various features observed in human BCC lesions and in mouse BCC models (14, 15, 31). First, these nodular structures contained predominantly clusters of cells with intensely stained nuclei and a high nucleus to cytoplasm ratio, surrounded by stromal fibroblasts (Fig. 2 E and G–I). In addition, each nodule appeared to be enclosed by a peripheral layer of palisading cells (Fig. 2 E and G–I). Furthermore, the nodules showed features of retracting spaces (Fig. 2 E and G–I), commonly observed when preparing histological sections of BCC samples. Finally, through immunocytochemical studies, the nodular structures were seen to be associated with relatively high levels of CK14, a marker for basal keratinocytes (Fig. 2G), and low levels of CK10, and loricrin, markers for more differentiated keratinocytes (Fig. 2 H and I), again typical of what has been reported for human BCC (31).
CUR61414 Blocks the Formation and Induces the Regression of Basaloid Lesions.
When added with Shh to embryonic skin punches, CUR61414 blocked Hh-induced signaling in a dose-dependent manner (Fig. 2 B and C and Fig. 8, which is published as supporting information on the PNAS web site). This was seen in decreased β-galactosidase activity and in decreased levels of Gli-1 message (data not shown). Inhibition of Hh signaling led to complete prevention of lesion formation. This was confirmed by careful histological examination of the cultured skin (Fig. 2F).
Next, we examined the effect of CUR61414 on basaloid nests that were preestablished by 6 days of skin punch treatment with Shh (Fig. 3A). The punches were exposed to either vehicle or CUR61414 in the continued presence of Shh for an additional 4 days. Histological analysis showed that CUR61414 significantly decreased the size and number of basaloid structures so that they became barely detectable (Fig. 3B). Importantly, this compound did not appear to affect normal cells in the epidermis and dermis (Fig. 3B). Accordingly, the expression profiles of normal epidermal markers appeared unchanged in compound-treated skin (Fig. 9, which is published as supporting information on the PNAS web site),
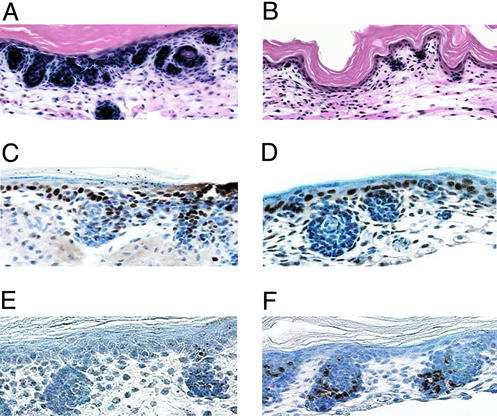
Figure 3.
CUR61414 blocks the response of embryonic skin to Hh. (A and B) CUR61414 causes the regression of preformed BCC-like structures. H&E counterstained sections from skin punches after a 6-day treatment with ShhOCT followed by a 4-day treatment with ShhOCT (A) and [ShhOCT + CUR61414 (1 μM)] (B). (C and D) CUR61414 inhibits proliferation of cells in the Shh-induced basaloid nests. PCNA staining of sections from skin punches, after a 6-day Hh treatment followed by 48 h with vehicle (C) or CUR61414 (D; 1 μM). (E and F) CUR61414 induces apoptosis in the Shh-induced basaloid nests. TUNEL staining of adjacent sections from the same skin punches as above; vehicle (E) or CUR61414 (F; 1 μM). For the mitotic and apoptotic indices, see Table 1.
To investigate how CUR61414 induced regression of the basaloid nests, skin punches with established lesions were exposed to this compound for only 48 h to determine effects on proliferation and apoptosis of cells inside the nests and in the remaining normal areas of skin. Proliferation in the basaloid nests was significantly reduced after CUR61414 treatment (Fig. 3 C and D; Table 1), whereas the proliferation of normal basal keratinocytes appeared unaffected (Fig. 3D). In addition, the number of apoptotic nuclei within those structures was significantly elevated in the compound-treated samples (Fig. 3 E and F; Table 1). Again, hardly any apoptotic cells were observed outside of the basaloid nests. (Fig. 3F). The caspase inhibitor zVAD blocked CUR61414-induced cell death in these lesions, but did not affect CUR61414's inhibition of proliferation in the basaloid nests (data not shown). These data suggest that specific inhibition of proliferation and growth arrest followed by apoptosis result in disappearance of the treated BCC-like basaloid lesions.
Table 1.
CUR61414 blocks proliferation and induces apoptosis in basaloid lesions in mouse embryonic skin punches
| Treatment of skin punches | Mitotic index, %* | Apoptotic index, %† |
|---|---|---|
| Vehicle‡ | 35.63 ± 5.38 | 4.83 ± 0.92 |
| CUR61414 (1 μM) | 10.46 ± 4.13 | 31.75 ± 4.17 |
| CUR61414 (5 μM) | 7.71 ± 2.63 | 34.33 ± 6.71 |
Skin punches were first treated with ShhOCT followed by treatment with [ShhOCT + Vehicle] or [ShhOCT + CUR61414] for another 48 h. The average number of cells per lesion was 24 (100%).
Percent PCNA-labeled cells per lesion.
Percent TUNEL-labeled cells per lesion.
0.1–0.5% DMSO.
CUR61414 Shrinks UV-Induced Basaloid Lesion in Adult Mouse Skin.
Finally, we wanted to test the efficacy of the Hh inhibitor against basaloid lesions induced when Ptch+/− heterozygous mice are exposed to UV light (15). These mice were UV irradiated for 6–9 months, producing many microscopic BCC-like basaloid lesions throughout their skin, as reported by Aszterbaum et al. (15). To test compounds, skin punches were taken from the UV-irradiated mice and placed in culture.
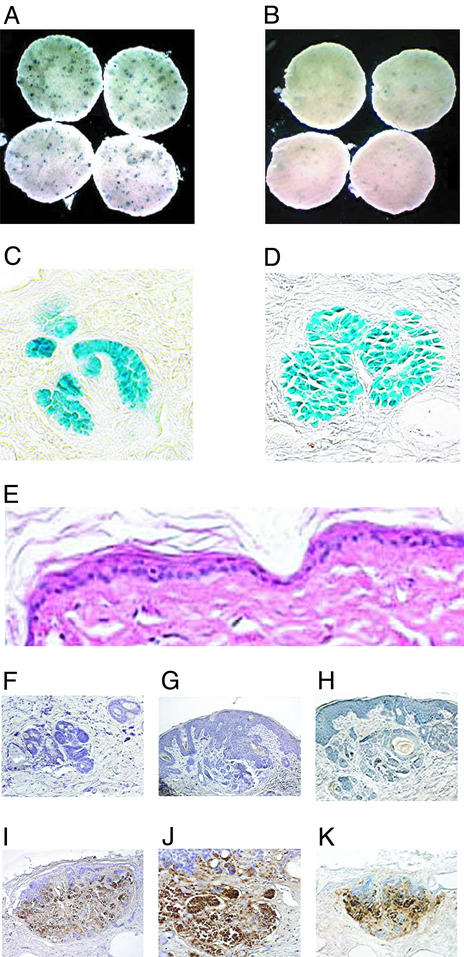
The small BCC-like lesions induced by UV treatment (Fig. 4 A and B; blue spots) were morphologically similar to the Hh-induced basaloid nests in embryonic skin (Fig. 4 C and D). They maintained an elevated level of Hh signaling and remained intact after several days in culture, as reflected in the continued high levels of β-galactosidase activity in the basaloid nests (Fig. 4 C and D). As with the embryonic skin punch assay, elevated Hh signaling, reflected by intense X-gal staining, was predominantly associated with the basaloid nests, and not with the surrounding stroma (Fig. 4 C and D). Treatment with CUR61414 caused regression of these lesions, as observed by both X-gal staining (Fig. 4 A and B; Table 2) and histological analysis (data not shown). Regression in the CUR61414-treated punches appeared to be the result of massive cell death, as a significant increase in apoptotic nuclei was observed in basaloid nests after treatment (Fig. 4 F–K). Again, no overt toxicity or noticeably increased apoptosis was observed in the skin surrounding the lesions (Fig. 4E).
Figure 4.
CUR61414 induces regression of basaloid lesions in skin punches from UV-irradiated Ptch+/−-heterozygous mice. (A and B) Whole-mount X-gal staining of punches from UV-treated skin. After 6 days in culture, punches were treated for an additional 4 days with vehicle (0.5% DMSO) (A) or CUR61414 (5 μM) (B). This resulted in a significant reduction of the number of microscopic lesions (blue spots; see also Table 2). (C and D) Histological analysis of sections from the X-gal-stained skin punches, after UV-treatment: eosin counterstaining of sections from skin punches before (C) and after (D) culture in vitro. (E) H&E staining of a section from a CUR61414-treated skin punch; no overtoxicity is observed. (F–K) TUNEL staining of sections from vehicle-treated (F–H) or CUR61414-treated (I–K) skin punches, containing UV-induced basaloid lesion. A significant increase in apoptosis is observed after CUR61414 treatment.
Table 2.
CUR61414 induces regression of UV-induced basaloid lesions in skin from adult Ptc+/−-lacZ heterozygote transgenic mice
| Experiment number | Treatment of skin punches* | Number of basaloid lesions† |
|---|---|---|
| 1 | Vehicle | 50.50 ± 5.30 |
| CUR61414 | 15.75 ± 5.70 | |
| 2 | Vehicle | 12.00 ± 1.41 |
| CUR61414 | 4.00 ± 0.82 | |
| 3 | Vehicle | 20.25 ± 6.20 |
| CUR61414 | 9.75 ± 3.60 |
Four-millimeter skin punches were generated from the irradiated mice and cultured for 96 h in vitro, with vehicle (0.5% DMSO) or CUR61414 (5 μM).
Average number of lesions across four skin punches.
Discussion
A substantial body of information suggests that the Hh signaling pathway, a key regulator of embryonic development, is involved in the initiation of BCC (32, 33), the most common cancer in the western world. The data consistently support the view that prolonged and uncontrolled stimulation of this pathway promotes basal cell proliferation and formation of the generally nonmetastatic basaloid nests that define BCC. Thus, we were interested in testing whether potent small molecule inhibitors of this pathway could be used to treat this particular type of cancer, either by causing tumor regression or, at least, by preventing tumor recurrence or growth of new tumors. That this might indeed occur is supported by a recent publication by Berman et al. (20), showing that cyclopamine can inhibit the growth of medulloblastoma cells whose growth is stimulated by activation of the Hh signaling pathway.
To accomplish this, we set up a screen for inhibitors that act downstream of Ptch-1, the protein most commonly mutated in this disease. We identified several different chemical classes of inhibitors, each of which strongly inhibits Hh signaling in all test systems to which they have been applied to date. CUR61414 is a synthetic inhibitor that specifically binds to and antagonizes the activity of Smo in the Hh signaling pathway (24). Similarly, in a recent study by Chen et al. (28) several other classes of small synthetic modulators of Hh signaling were presented that bind to Smo.
CUR61414 has physiochemical properties (e.g., size, ease of synthesis, solubility, etc.) that make it a desirable candidate for drug development. In addition, CUR61414 has certain pharmacological properties not described in this paper, such as prolonged inactivation of the Hh pathway after a single application and lack of observable toxicity in preclinical testing that also make it a suitable drug candidate.
To validate the Hh inhibitors in a more BCC-like setting, we set up two novel skin punch culture systems. One is based on treating embryonic skin punches for several days with Shh protein, thereby providing a tissue culture model similar in most regards to the transgenic mouse model described by Oro et al. (14). This culture system is easy to set up, and BCC-like basaloid nests, with characteristic morphology and markers, form in a few days. Compounds can be readily tested under these circumstances. When administered together with Hh protein, Hh antagonists were able to block the formation of the lesions, but had no effect on normal keratinocytes or stromal cells in the skin. Hh inhibitors were also tested for their ability to cause regression of preexisting lesions. CUR61414 was able to decrease the rate of proliferation of cells within the tumors significantly without affecting proliferation outside of the lesions. Correspondingly, CUR61414 increased the rate of apoptosis of tumor cells without causing an obvious increase in apoptosis of normal skin cells. Additionally, no nonspecific toxicity was seen even after more than a week of treatment with CUR61414. This finding suggests that CUR61414 acts as an anti-proliferative agent affecting specifically cells in which proliferation is driven by activation of the Hh pathway (as in the basaloid lesions). These cells, prevented from dividing, die by apoptosis. However, normal skin cells, where proliferation is not dependent on Hh signaling, are not affected by the presence of CUR61414. These effects are quite different from what would be observed if skin punches were treated with an anti-mitotic agent, such as 5-flurouracil.
Although the embryonic skin punch assay offers many advantages for testing compounds in vitro, the Hh-induced basaloid lesions are unlikely to have accumulated additional mutations commonly found in human BCC, notably p53 and Ras (11, 34, 35). Some of those mutations, however, have been found in a mouse BCC model, as described by Aszterbaum et al. (15). Ptch+/− mice were subjected to long periods of UV irradiation, resulting in small, but typical, BCCs throughout their skin. To rule out the possibility that the mutations residing outside the Hh signaling pathway might influence the efficacy of the Hh inhibitor, skin punches from the UV-irradiated mice were placed in culture under conditions in which they maintained their phenotypes for several days. Again, CUR61414 was able to induce apoptosis within the BCC-like lesions rapidly and selectively without having toxic or other nonspecific effects on normal skin cells. Future work will be needed to confirm that these compounds are effective when applied to lesions in vivo.
Finally, as the involvement of the signaling pathways that regulate embryonic development (e.g., Hedgehog, Wnt, transforming growth factor β; refs. 36–38), in the control of tumor growth becomes clearer, the convergence of developmental biology and oncology will undoubtedly be an area of increasing focus. It will be important to determine whether it is possible to cause regression of tumors in which secondary mutations have accumulated by inhibiting the primary pathways involved in their initiation. This would represent a truly novel way of treating certain types of cancer.
Supplementary Material
Acknowledgments
We thank John Lydon and Ricky Sanchez for histology assistance, Jean Flanagan, Sarah Leiker, and JoAnn Wicker for technical help, David Bumcrot for valuable discussions, and Doug Barker and Jane LaLonde for editorial assistance.
Abbreviations
- BCC
basal cell carcinoma
- Hh
Hedgehog
- Shh
Sonic hedgehog
- Ptch-1
patched-1
- Smo
smoothened
- CK
cytokeratin
- H&E
hematoxylin and eosin
- ShhOCT
lipid-modified (octylated) form of Shh
- X-gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP end labeling
- PCNA
proliferating cell nuclear antigen
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ingham P W, McMahon A P. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 2.Chiang C, Litingtung Y, Lee E, Young K E, Corden J L, Westphal H, Beachy P A. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 3.St-Jacques B, Hammerschmidt M, McMahon A P. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitgood M J, Shen L, McMahon A P. Curr Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- 5.Hahn H, Wicking C, Zaphiropoulous P G, Gailani M R, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden A B, Gillies S, et al. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 6.Johnson R L, Rothman A L, Xie J, Goodrich L V, Bare J W, Bonifas J M, Quinn A G, Myers R M, Cox D R, Epstein E H, Jr, Scott M P. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 7.Raffel C, Jenkins R B, Frederick L, Hebrink D, Alderete B, Fults D W, James C D. Cancer Res. 1997;57:842–845. [PubMed] [Google Scholar]
- 8.Xie J, Murone M, Luoh S M, Ryan A, Gu Q, Zhang C, Bonifas J M, Lam C W, Hynes M, Goddard A, et al. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 9.Miller D L, Weinstock M A. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 10.Gorlin R J. Medicine (Baltimore) 1987;66:98–113. doi: 10.1097/00005792-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Gailani M R, Leffell D J, Ziegler A, Gross E G, Brash D E, Bale A E. J Natl Cancer Inst. 1996;88:349–354. doi: 10.1093/jnci/88.6.349. [DOI] [PubMed] [Google Scholar]
- 12.Gailani M R, Stahle-Backdahl M, Leffell D J, Glynn M, Zaphiropoulos P G, Pressman C, Unden A B, Dean M, Brash D E, Bale A E, Toftgard R. Nat Genet. 1996;14:78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 13.Dahmane N, Lee J, Robbins P, Heller P, Ruiz i Altaba A. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 14.Oro A E, Higgins K M, Hu Z, Bonifas J M, Epstein E H, Jr, Scott M P. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 15.Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit P E, Scott M P, Epstein E H., Jr Nat Med. 1999;5:1285–1289. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson M, Unden A B, Krause D, Malmqwist U, Raza K, Zaphiropoulos P G, Toftgard R. Proc Natl Acad Sci USA. 2000;97:3438–3443. doi: 10.1073/pnas.050467397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui C C, Dlugosz A A. Nat Genet. 2000;24:216–217. doi: 10.1038/73417. [DOI] [PubMed] [Google Scholar]
- 18.Fan H, Khavari P A. J Cell Biol. 1999;147:71–76. doi: 10.1083/jcb.147.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taipale J, Chen J K, Cooper M K, Wang B, Mann R K, Milenkovic L, Scott M P, Beachy P A. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 20.Berman D M, Karhadkar S S, Hallahan A R, Pritchard J I, Eberhart C G, Watkins D N, Chen J K, Cooper M K, Taipale J, Olson J M, Beachy P A. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 21.Taylor F R, Wen D, Garber E A, Carmillo A N, Baker D P, Arduini R M, Williams K P, Weinreb P H, Rayhorn P, Hronowski X, et al. Biochemistry. 2001;40:4359–4371. doi: 10.1021/bi002487u. [DOI] [PubMed] [Google Scholar]
- 22.Roelink H, Porter J A, Chiang C, Tanabe Y, Chang D T, Beachy P A, Jessell T M. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- 23.Ericson J, Morton S, Kawakami A, Roelink H, Jessell T M. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 24.Frank-Kamenetsky M, Zhang X M, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang F Y, Jones S, Shulok J, Rubin L L, Porter J A. J Biol. 2002;1:10.1–10.19. doi: 10.1186/1475-4924-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper M K, Porter J A, Young K E, Beachy P A. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 26.Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell T M, Briscoe J. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- 27.Briscoe J, Sussel L, Serup P, Hartigan-O'Conner D, Jessell T M, Rubenstein J L, Ericson J. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- 28.Chen J K, Taipale J, Young K E, Maiti T, Beachy P A. Proc Natl Acad Sci USA. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J K, Taipale J, Cooper M K, Beachy P A. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan H, Oro A E, Scott M P, Khavari P A. Nat Med. 1997;3:788–792. doi: 10.1038/nm0797-788. [DOI] [PubMed] [Google Scholar]
- 31.Miller S J. J Am Acad Dermatol. 1991;24:1–13. doi: 10.1016/0190-9622(91)70001-i. [DOI] [PubMed] [Google Scholar]
- 32.Toftgard R. Cell Mol Life Sci. 2000;57:1720–1731. doi: 10.1007/PL00000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saldanha G J. J Pathol. 2001;193:427–432. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH815>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.van der Riet P, Karp D, Farmer E, Wei Q, Grossman L, Tokino K, Ruppert J M, Sidransky D. Cancer Res. 1994;54:25–27. [PubMed] [Google Scholar]
- 35.van der Schroeff J G, Evers L M, Boot A J, Bos J L. J Invest Dermatol. 1990;94:423–425. doi: 10.1111/1523-1747.ep12874504. [DOI] [PubMed] [Google Scholar]
- 36.Taipale J, Beachy P A. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 37.Bienz M, Clevers H. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 38.Wakefield L M, Roberts A B. Curr Opin Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.