Abstract
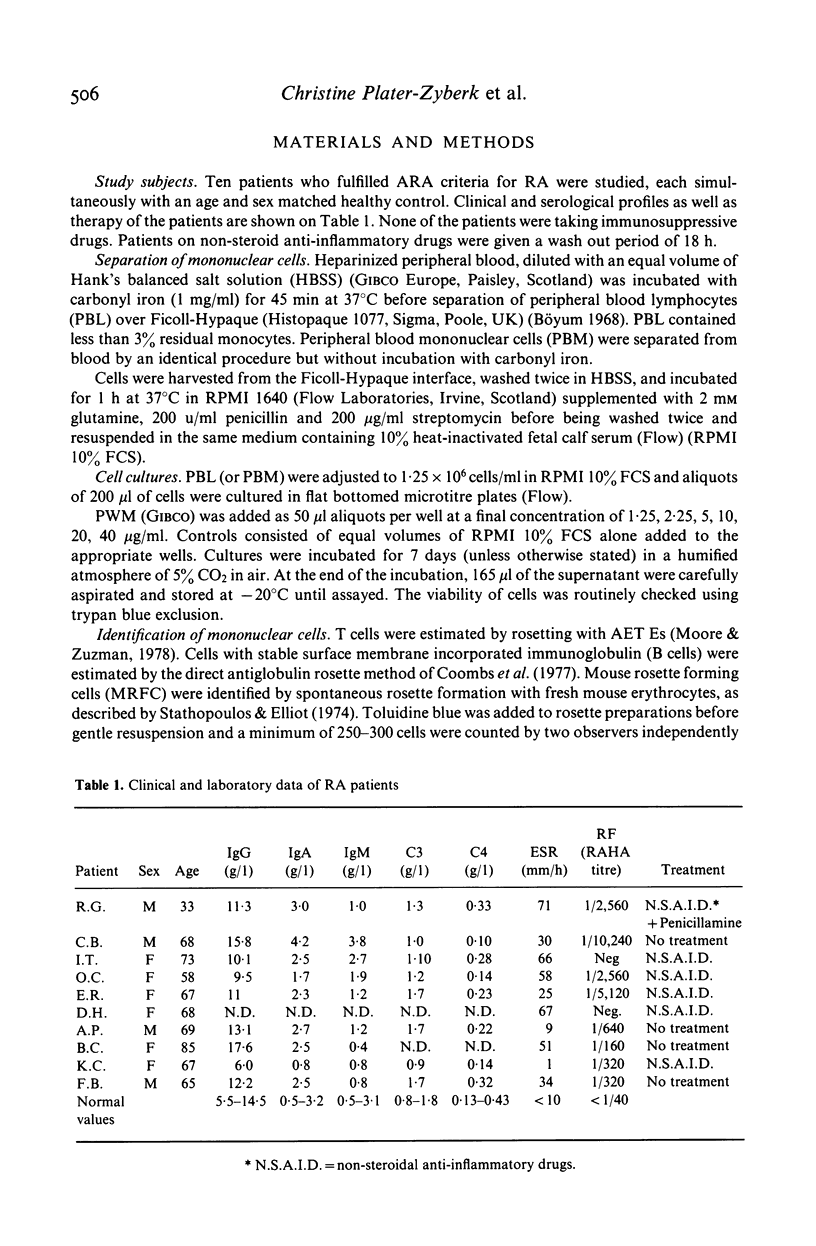
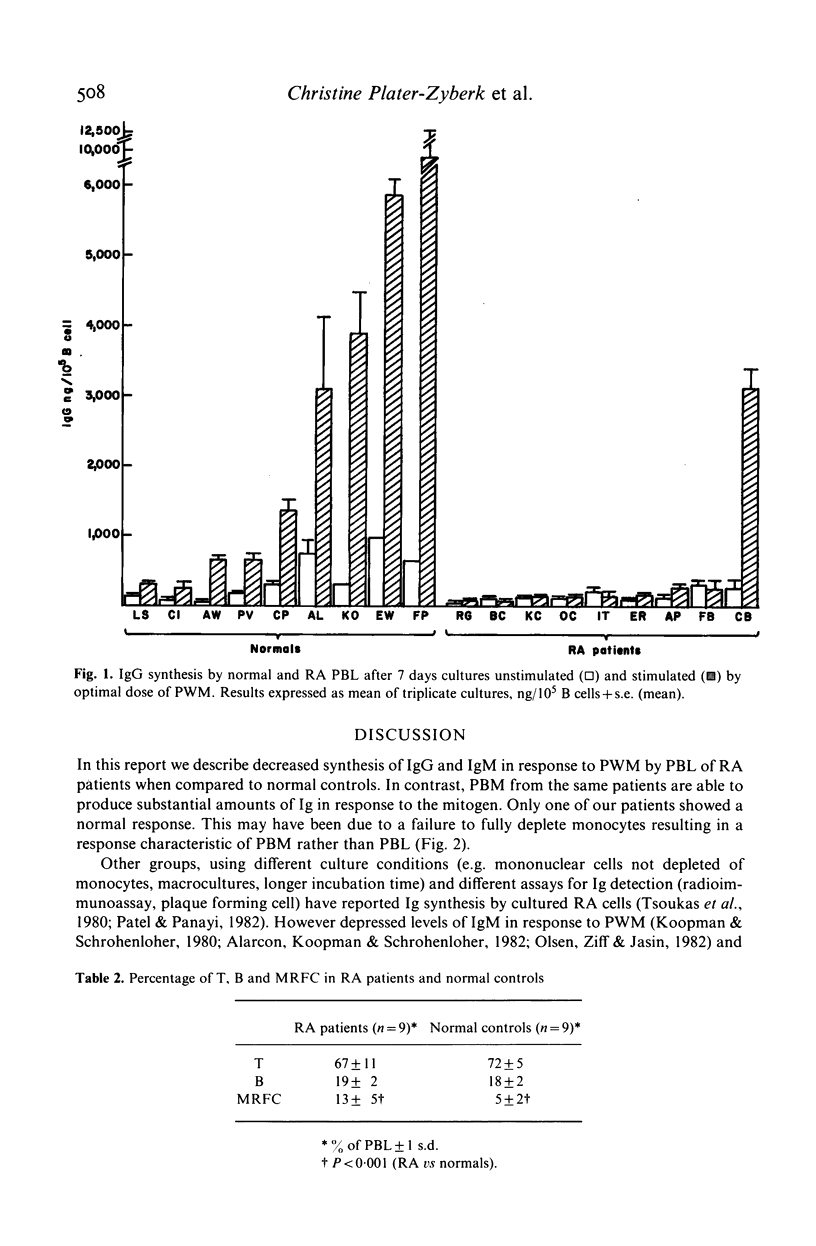
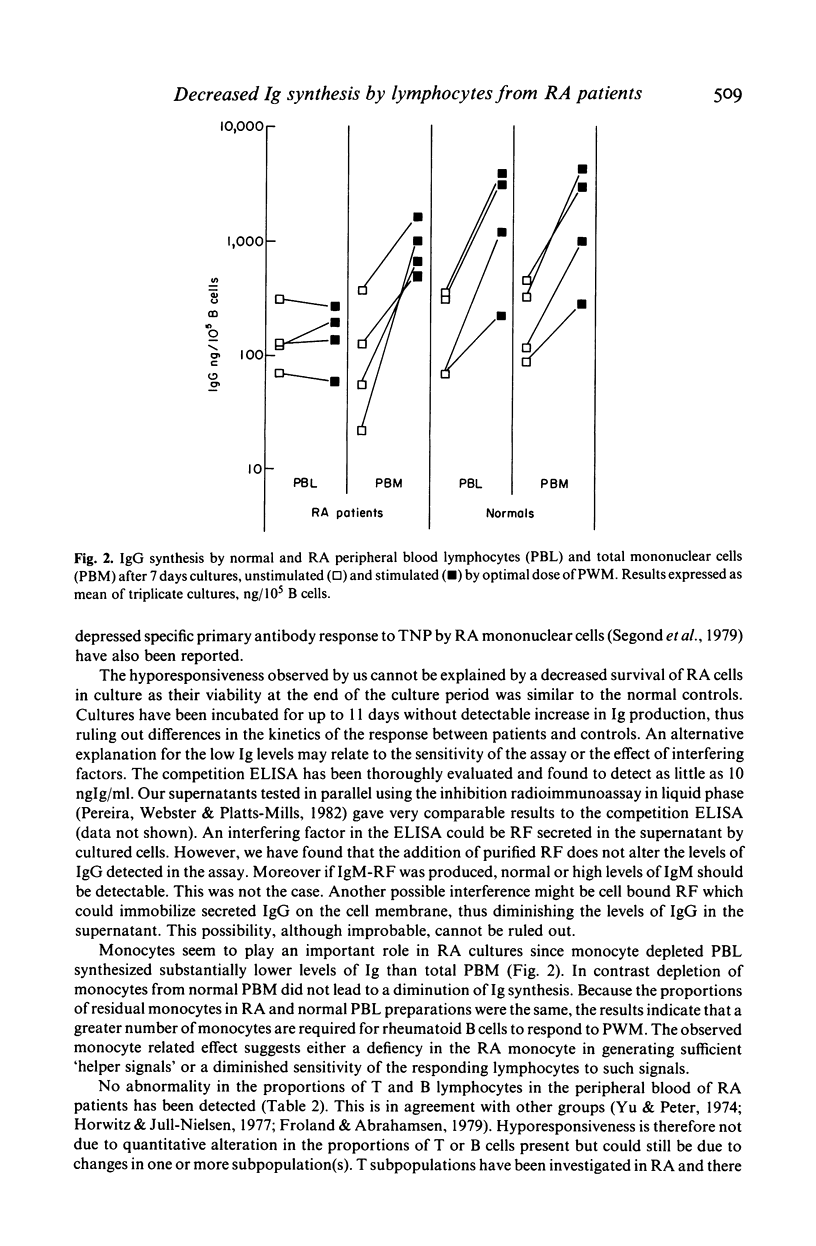
We have investigated B cell function in nine patients with rheumatoid arthritis (RA) compared to sex and age matched controls in a pokeweed mitogen driven system. Levels of IgG and IgM synthesized in the supernatant were measured by a competition ELISA. We have found that cultured mononuclear cells from RA patients showed a defective Ig synthesis when depleted of monocytes. In contrast RA mononuclear cells not depleted of monocytes produced substantial levels of Ig after stimulation by the mitogen. The percentages of T and B lymphocytes in the peripheral blood of RA patients were normal; however, an increased number of lymphocytes formed rosettes with mouse erythrocytes indicating an abnormality in the B cell pool. These results demonstrate defective in vitro immunoglobulin synthesis by RA lymphocytes and show the importance of monocytes in this culture system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Lindsley H. B., Racela L. S., Pascual E., Hassanein K. M. Suppressor T cell dysfunction and anti-suppressor cell antibody in active early rheumatoid arthritis. J Rheumatol. 1981 Jan-Feb;8(1):9–18. [PubMed] [Google Scholar]
- Alarcón G. S., Koopman W. J., Schrohenloher R. E. Differential patterns of in vitro IgM rheumatoid factor synthesis in seronegative and seropositive rheumatoid arthritis. Arthritis Rheum. 1982 Feb;25(2):150–155. doi: 10.1002/art.1780250206. [DOI] [PubMed] [Google Scholar]
- Ault K. A., Towle M. Human B lymphocyte subsets. I. IgG-bearing B cell response to pokeweed mitogen. J Exp Med. 1981 Feb 1;153(2):339–351. doi: 10.1084/jem.153.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P., Burmester G. R., Ledwoch A., Urban C., Kalden J. R. Autologous and allogeneic MLC-reactivity in patients with rheumatoid arthritis. J Clin Lab Immunol. 1981 Jul;6(1):27–33. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Coombs R. R., Wilson A. B., Eremin O., Gurner B. W., Haegert D. G., Lawson Y. A., Bright S., Munro A. J. Comparison of the direct antiglobulin rosetting reaction with the mixed antiglobulin rosetting reaction for the detection of immunoglobulin on lymphocytes. J Immunol Methods. 1977;18(1-2):45–54. doi: 10.1016/0022-1759(77)90157-0. [DOI] [PubMed] [Google Scholar]
- Davey F. R., Kurec A. S. Mouse rosette positive B cells stimulate poorly in the autologous and allogeneic mixed lymphocyte reaction. Clin Exp Immunol. 1982 Jul;49(1):193–199. [PMC free article] [PubMed] [Google Scholar]
- Forbes I. J., Zalewski P. D., Valente L., Gee D. Two maturation-associated mouse erythrocyte receptors of human B cells. I. Identification of four human B-cell subsets. Clin Exp Immunol. 1982 Feb;47(2):396–404. [PMC free article] [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Gupta S., Good R. A., Siegal F. P. Rosette formation with mouse erythrocytes. III. Studies in patients with primary immunodeficiency and lymphoproliferative disorders. Clin Exp Immunol. 1976 Nov;26(2):204–213. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Good R. A., Siegal F. P. Rosette-formation with mouse erythrocytes. II. A marker for human B and non-T lymphocytes. Clin Exp Immunol. 1976 Aug;25(2):319–327. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Pahwa R., O'Reilly R., Good R. A., Siegal F. P. Ontogeny of lymphocyte subpopulations in human fetal liver. Proc Natl Acad Sci U S A. 1976 Mar;73(3):919–922. doi: 10.1073/pnas.73.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman P. B., Stobo J. D. Specificity and function of a human autologous reactive T cell. J Exp Med. 1979 Jun 1;149(6):1537–1542. doi: 10.1084/jem.149.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. A., Juul-Nielsen K. Human blood L lymphocytes in patients with active systemic lupus erythematosus, rheumatoid arthritis and scleroderma: a comparison with T and B cells. Clin Exp Immunol. 1977 Dec;30(3):370–378. [PMC free article] [PubMed] [Google Scholar]
- Keystone E. C., Gladman D. D., Buchanan R., Cane D., Poplonski L. Impaired antigen-specific suppressor cell activity in patients with rheumatoid arthritis. Arthritis Rheum. 1980 Nov;23(11):1246–1250. doi: 10.1002/art.1780231103. [DOI] [PubMed] [Google Scholar]
- Koopman W. J., Schrohenloher R. E. Enhanced in vitro synthesis of IgM rheumatoid factor in rheumatoid arthritis. Arthritis Rheum. 1980 Sep;23(9):985–992. doi: 10.1002/art.1780230904. [DOI] [PubMed] [Google Scholar]
- Lucivero G., Lawton A. R., Cooper M. D. Rosette formation with mouse erythrocytes defines a population of human B lymphocytes unresponsive to pokeweed mitogen. Clin Exp Immunol. 1981 Jul;45(1):185–190. [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Stacey M. C. Further characterization of the human autologous mixed leukocyte reaction (MLR). J Immunol. 1981 Feb;126(2):729–734. [PubMed] [Google Scholar]
- Meijer C. J., Lafeber G. J., Cnossen J., Damsteeg M. G., Cats A. T lymphocyte subpopulations in rheumatoid arthritis. J Rheumatol. 1982 Jan-Feb;9(1):18–24. [PubMed] [Google Scholar]
- Olsen N., Ziff M., Jasin H. E. In vitro synthesis of immunoglobulins and IgM-rheumatoid factor by blood mononuclear cells of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):59–66. doi: 10.1007/BF00541247. [DOI] [PubMed] [Google Scholar]
- Palacios R., Ruíz-Arguelles A., Alarcón-Segovia D. Human post-thymic precursor cells in health and disease. IX. Immunoregulatory T cell circuits in peripheral blood of patients with rheumatoid arthritis. Clin Exp Immunol. 1981 Mar;43(3):473–477. [PMC free article] [PubMed] [Google Scholar]
- Pereira S., Webster D., Platts-Mills T. Immature B cells in fetal development and immunodeficiency: studies of IgM, IgG, IgA and IgD production in vitro using Epstein-Barr virus activation. Eur J Immunol. 1982 Jul;12(7):540–546. doi: 10.1002/eji.1830120703. [DOI] [PubMed] [Google Scholar]
- Room G. R., Plater-Zyberk C., Clarke M. F., Maini R. N. B-lymphocyte subpopulation which forms rosettes with mouse erythrocytes increased in rheumatoid arthritis. Rheumatol Int. 1982;2(4):175–178. doi: 10.1007/BF00286140. [DOI] [PubMed] [Google Scholar]
- Segond P., Delfraissy J. F., Galanaud P., Wallon C., Massias P., Dormont J. Depressed primary in vitro antibody response in rheumatoid arthritis. Clin Exp Immunol. 1979 Aug;37(2):196–204. [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos G., Elliott E. V. Formation of mouse or sheep red-blood-cell rosettes by lymphocytes from normal and leukaemic individuals. Lancet. 1974 Apr 6;1(7858):600–601. doi: 10.1016/s0140-6736(74)92655-5. [DOI] [PubMed] [Google Scholar]
- Tsoukas C. D., Carson D. A., Fong S., Pasquali J. L., Vaughan J. H. Cellular requirements for pokeweed mitogen-induced autoantibody production rheumatoid arthritis. J Immunol. 1980 Sep;125(3):1125–1129. [PubMed] [Google Scholar]
- Vaughan J. H. Dunlop-Dottridge lecture. Rehumatoid arthritis, rheumatoid factor and the Epstein-Barr virus. J Rheumatol. 1979 Jul-Aug;6(4):381–388. [PubMed] [Google Scholar]
- Venables P. J., Erhardt C. C., Maini R. N. Antibodies to extractable nuclear antigens in rheumatoid arthritis: relationship to vasculitis and circulating immune complexes. Clin Exp Immunol. 1980 Jan;39(1):146–153. [PMC free article] [PubMed] [Google Scholar]
- Veys E. M., Hermanns P., Schindler J., Kung P. C., Goldstein G., Symoens J., Van Wauwe J. Evaluation of T cell subsets with monoclonal antibodies in patients with rheumatoid arthritis. J Rheumatol. 1982 Jan-Feb;9(1):25–29. [PubMed] [Google Scholar]
- Yu D. T., Peter J. B. Cellular immunological aspects of rheumatoid arthritis. Semin Arthritis Rheum. 1974 Fall;4(1):25–52. doi: 10.1016/0049-0172(74)90016-x. [DOI] [PubMed] [Google Scholar]



