Abstract
In female mammals, it remains controversial whether maternal diet and particularly the source and availability of energy can influence sex of offspring born. Outbred female mice were fed ad libitum from 30 days to ≈45 wk of age on defined, complete diets that differed only in their relative content of fat and carbohydrate to determine whether calorie source influenced litter size and sex ratio of pups. Diet 1 (very high in saturated fat, VHF) provided 60% of calories as fat, mainly lard. Diet 2 (low in saturated fat, LF) was low in fat (10% of calories) but high in carbohydrate. Mice delivered four litters of pups, resulting in a total of 1,048 young born over 108 pregnancies. Gestation length and litter size did not differ between VHF and LF groups and did not change as mice aged. Sex ratio of pups (fraction male) born to mothers on VHF diet was unusually high (0.67) and to mothers on LF diet very low (0.39) over litters 2, 3, and 4. This skewing of sex ratio was related to diets fed and not to body mass of mothers. Age of mothers was an important variable, however. Mice that were first bred at 10 wk of age delivered similar numbers of sons and daughters, whereas virgin mice bred later than 20 wk of age produced litters that were skewed toward males or females according to diet. The data show that the source of calories provided in a nutritionally complete diet to mature female mice can influence sex of offspring born.
Many invertebrate and some avian species can adjust the sex ratio (usually defined as proportion male) among their progeny in a highly predictable manner depending on prevailing environmental conditions and the associated relative costs and benefits of producing more offspring of one sex than the other (1–7). Sex ratio adjustments seem to contribute to parental fitness by ensuring that parental genes are transmitted most efficiently to future generations at the least cost. Although there are numerous reports of sex ratio variation in mammals in relationship to factors such as food availability and competition for resources (8), and evidence that some of the changes might be adaptive and in accordance with evolutionary theory, this area remains controversial (8). Perhaps the best-known examples of major changes in sex ratio in mammals have been reported for various species of deer, either in the wild or under some form of confinement (8–11). Trivers and Willard (12) in their sex allocation theory predicted that, in such wild polygynous species, where a small proportion of males sire most of the young yet invest little in their care, females in the best body condition would be anticipated to produce more sons than daughters, because such male offspring would be more likely to join the class of elite breeder males when they reached adulthood. Conversely, mothers in poorer body condition would be expected to invest more in female young, because their sons would have a relatively lower chance of reproductive success than their daughters. Although relatively straightforward in concept, this theory has been difficult to prove, with sex ratios sometimes deviating from the direction predicted (8, 10). An explanation for the inconsistencies is that variables other than maternal body condition, including population density (10), influence sex ratio adjustment. In wild populations, in particular, group sizes are often too small for rigorous statistical evaluation and the life histories of the mothers too variable for predictable patterns to emerge (6–8).
Surprisingly few studies aimed at examining whether nutrition of the mother can affect sex ratio have been carried out under laboratory conditions. Rodents, like most mammals, tend to produce roughly equivalent numbers of sons and daughters (13–15), although litters with marked imbalances in sex ratio can occur spontaneously. When rodents are food-restricted, however (13, 16–18), or are provided diets suboptimal in essential fatty acids (19) or protein (20), they tend to produce small, female-biased litters, although there is at least one report where sex ratio was not altered in mice that were deprived of adequate food (21). Stresses other than food restriction can also reduce the fraction of males born to rodents (22–25). When ad libitum-fed hamsters are exposed to an aggressive female, for example, their resulting litters become skewed toward females and are smaller than in controls due to the loss of male fetuses after implantation (26). Pregnant mice and rats seem to respond similarly to stress imposed during pregnancy, perhaps reflecting the selective prenatal vulnerability of male fetuses observed in many mammals, including humans (27, 28). Nutritionally complete diets provided ad libitum to rodents housed under the standard conditions approved for laboratory species have, in general, not been tested experimentally for their effects on sex of pups. One study that retrospectively analyzed the breeding records of mice strains maintained at the Jackson Laboratory between 1959 and 1966 noted no major deviations in sex ratio accompanying a switch in commercial diets, which varied slightly in relative fat and protein content (29). In another study, laboratory and captive wild mice produced more male- than female-biased litters in the spring and summer months at a time when food would be more abundant in the wild (30). This observation suggests that seasonal shifts in reproductive productivity are inexplicably retained during captivity when the usual environmental cues would be absent. In the experiments that follow, we have chosen to examine the effects of two complete diets (ref. 31; Table 1) that differ markedly in their sources of dietary energy on the sex of offspring born to female NIH Swiss mice. Diet 1 was low in saturated fat (LF diet), with the majority of calories provided as sugars and complex carbohydrate. The second was very high in saturated fat (VHF diet), with most energy provided as lard (Table 1). The goal was to determine whether these diets could influence the sex ratio of pups born.
Table 1.
Relative energy content (kcal%) of major nutrients in mouse diets
| Diet | D12450B* (LF) | D12492* (VHF) | Purina 5015 (CLC)† |
|---|---|---|---|
| Protein | 20 | 20 | 18 |
| Carbohydrates | |||
| Starch | 31 | 0 | 51 |
| Maltodextrin | 4 | 13 | NS |
| Sucrose | 35 | 7 | 1 |
| Total carbohydrates | 70 | 20 | 56 |
| Fats | |||
| Soybean oil | 6 | 6 | NS |
| Lard | 4 | 54 | NS |
| Total fat | 10 | 60 | 26 |
NS, Not specified.
Defined Research Diet (Research Diets, New Brunswick, NJ) with equivalent amounts of casein, cellulose, minerals, and vitamin mixes (31). D12450B diet had a caloric density of 3.8 kcal/g, and D12492 diet had a caloric density of 5.2 kcal/g.
Complete Life Cycle; 4.4 kcal/g.
Methods
Animals.
In the first experiment, 8- to 10-wk-old NIH Swiss mice (Harlan, Madison, WI) were bred to stud males. After weaning at 21 days, 16 female offspring were maintained on a regular chow diet, Purina 5001 (Purina), until they were 30 days of age and then randomly assigned to two groups of eight, and fed either the LF or VHF diets (Research Diets, New Brunswick, NJ; Table 1) continuously and ad libitum until the end of the study. The mice were housed in pairs. When they were 10, 20, 28, and 40 wk of age, the females were introduced to stud males (≈12 wk old). The females were housed individually from the end of the second week of pregnancy until they had weaned their litters. Pups were removed from their mothers 21 days after delivery. Mice were weighed every other day over the entire course of the study. A replicate study was initiated 4 wk after beginning the first to provide a total of 16 mothers in each dietary group. Because the results in the two studies were essentially identical, the data were combined for the statistical analysis summarized in Table 2.
Table 2.
Weight at conception, litter size, gestation length, fraction male pups, and number of male-biased litters over four successive pregnancies in mice maintained on the LF and VHF diets
| Treatment | Litter | n | Conception weight, g | Litter size | Pregnancy gestation length, days | Fraction male pups | No. of male-biased litters |
|---|---|---|---|---|---|---|---|
| LF | 1 | 15 | 20.8 ± 1.4 | 9.4 ± 1.7 | 20.0 ± 1.4 | 0.48 | 3 |
| 2 | 14 | 26.7 ± 2.2 | 10.8 ± 2.9 | 19.8 ± 1.4 | 0.45* | 4 | |
| 3 | 15 | 29.4 ± 5.0 | 9.1 ± 2.3 | 19.3 ± 1.5 | 0.35** | 1 | |
| 4 | 10 | 30.8 ± 2.3 | 9.1 ± 4.8 | 20.0 ± 1.4 | 0.38* | 0 | |
| VHF | 1 | 16 | 23.1 ± 2.2 | 9.5 ± 2.0 | 19.6 ± 2.1 | 0.51 | 10 |
| 2 | 15 | 30.6 ± 4.3 | 10.7 ± 2.8 | 18.8 ± 1.9 | 0.66** | 12 | |
| 3 | 14 | 35.7 ± 5.9 | 9.9 ± 2.3 | 20.0 ± 1.2 | 0.65** | 12 | |
| 4 | 9 | 38.0 ± 5.8 | 8.6 ± 4.3 | 19.9 ± 1.5 | 0.71** | 7 |
Females were housed in pairs. When they were ≈10, 20, 28, and 40 wk of age, they were introduced to a stud male. Females were housed individually from the end of week 2 of pregnancy until pups were weaned. Cannibalism, death of three females, and failure of some females to conceive account for the reduced litter numbers over the course of the study. Values for maternal weight at conception, litter size, and pregnancy length are means, with SD provided to indicate extent of variability. Weights of mothers on VHF and LF diets deviated significantly at second conception and thereafter. Sex ratio deviated significantly from 0.5;
, P < 0.05;
, P < 0.01.
In a second experiment, similar procedures were used, except the mice were bred just once when they were between 20 and 27 wk of age (n = 16 for each group). A group of control mice (n = 8) were also maintained continuously on the Purina 5015 chow diet and bred at age 10 and 20 wk.
Determination of Pup Gender.
Sex of pups was assessed by measuring anogenital distance (14) at day two after birth and confirmed at weaning. An additional analysis of pup gender was performed on ≈100 randomly chosen pups from tail DNA by PCR analysis for sex chromosome-specific sequences (32). No errors were noted in the anatomical gender assignments.
Statistical Procedures.
The effects of diet on litter size, maternal weight, gestation length, and sex ratio were tested by using mixed model procedures with a repeated measures design (33). Because each female had multiple correlated records within treatment, the pooled variance of values for the females in the two treatments was used to determine the effect of the diets. Parity and treatment by parity interactions were tested with residual error (34). Sex ratio (fraction male pups) for the VHF and LF groups was tested against the expected value of 0.5 by using a T-statistic (35).
Results
To determine whether the sex ratio of pups could be altered according to the source of calories provided in the diets, female mice were exposed to either the LF or VHF diets from age 30 days to ≈45 wk, during which time they delivered four litters of pups. These diets were identical in protein (casein), unsaturated fatty acid (provided in the form of soybean oil), and mineral content, but differed in their relative contents of carbohydrate and triglyceride (Table 1). The additional triglyceride in the VHF relative to the LF diet was in the form of lard. As a consequence of the disparity in fat content, the two diets had different caloric densities (3.8 vs. 5.2 kcal/g). Mice seemed to tolerate both diets well and showed no obvious ill effects. They gained weight and demonstrated normal fertility (discussed below).
Table 2 summarizes the data for 108 pregnancies and 1,048 pups born over four parities. The weights of the mothers on the two diets did not differ significantly between day 30, when the mice were first placed on the diets, and the time they were first bred (P > 0.1), but the VHF group was significantly heavier (P < 0.05) by the beginning of the second parity, and weights continued to deviate as the mice aged (Table 2, Fig. 1). By parity 4, the females on the VHF diet were ≈20% heavier (P < 0.001) than females on the LF diet (Table 2, Fig. 1), although there was considerable variation within groups. Litter sizes and lengths of pregnancy in the two groups were similar and did not change with parity, indicating that the diets did not suppress fertility in one group relative to the other. Pups born to mice on the two diets had similar weights at day 2 postpartum (data not shown). These values were similar to those observed with NIH Swiss mice fed Purina 5015 (data not shown). Together, the data suggest that reproductive performance had not been compromised on either experimental diet. Nor, was there any indication that the dams on the LF and VHF diets differed in their abilities to feed and care for their pups.
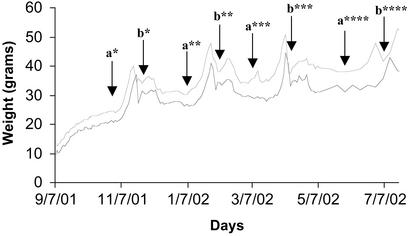
Figure 1.
Changes in mean body weight of female mice on the VHF and LF diets. The information is from one of the two studies from which the data in Table 2 were obtained. Two groups of eight mice were initially introduced to the diets when they were 30 days of age and maintained on the VHF (upper line) and LF (lower line) diets for ≈40 wk, during which time the mice delivered four sets of pups: a, male introduced; b, mean day of delivery; *, first parity 1; **, second parity; ***, third parity; ****, fourth parity. Pups were weaned at 21 days. The graph illustrates the rapid increase in body mass accompanying pregnancy, as well as the fall in weight after delivery and after weaning of pups. The number of mice successfully bred decreased from n = 8 for litters 1–3 to n = 5 for litter 4 when the mice in both groups proved difficult to breed. The results from the duplicate study were essentially indistinguishable from the one described here.
In contrast to the lack of difference in litter size and gestation length, mothers on the LF diet tended to produce female-biased litters and VHF mothers male-biased litters (Fig. 2; Table 2). This trend was noticeable at first parity but became more exaggerated at litters 2, 3, and 4. The overall sex ratio of total pups born within the two dietary groups over four parities differed markedly (P < 0.0001), with the LF group producing a preponderance of female pups and the VHF group more males. Although the sex ratio of pups born to LF mothers was not significantly different in first litters, it became skewed toward daughters at litters 2, 3, and 4 (Table 2). Conversely, the sex ratio of pups born after litter 1 to mothers on the VHF diet became highly male-biased. In contrast, comparably aged mice on the Purina 5015 diet gave birth to almost equal numbers of male and female pups at first and second parity (sex ratios 0.52 and 0.48, respectively). Studies on large numbers of inbred strains fed standard chow diets have also noted little change in sex ratio of pups with increased parity (29).
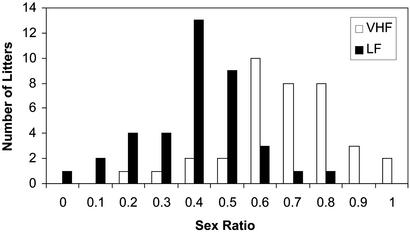
Figure 2.
Frequency distribution of sex ratio (fraction males) within litters at parity 2, 3, and 4 for the VHF and LF dietary groups in the experiment described in Table 2. Each of the bars on that graph represents the number of litters with a sex ratio within the range ±0.05 of the number on the x axis; i.e., a value of 0.1 means there were no VHF litters with sex ratio between 0.05 and 0.15, whereas for LF there were two. Similarly, there were eight litters with a ratio between 0.75 and 0.85 on the VHF diet and one on the LF diet. Two VHF litters were entirely male and one LF litter was entirely female.
Two immediate questions arise from these results. The first is whether the differences arose as an outcome of diet or maternal weight. For example, when mice were selected for body weight for over 40 generations, there was a slight increase in sex ratio as selection proceeded (36). The second question is whether the divergence of the sex ratio first observed at second pregnancy between mice on the LF and VHF diets was a parity or age effect. Question 1 was addressed by including weights of the mothers as a covariate in the statistical model. The analysis showed that diet, not maternal weight, was the main variable that determined the sex of the pups. This conclusion becomes evident if the fraction of male pups in the LF group at litter 4 (0.38) is compared with the same values for the VHF group at litter 2 (0.68). The mice had comparable body mass, yet produced litters widely divergent in sex ratio. Also, within dietary groups there was no relationship between the weights of individual mice and their tendencies to produce male- or female-biased litters.
To determine whether it was parity or the age of the mice that influenced the sex ratio of the pups, virgin females, maintained from day 30 on either the VHF or LF diets, were first bred after they had reached at least 20 wk of age, rather than 10 wk as in the first experiment (Table 3). Mothers on the VHF diet produced male-biased litters and a high fraction of males whereas the LF group birthed more females in mainly female-biased litters. Again, litter size and gestation length were unaffected by the diets.
Table 3.
Effect of diet on sex ratio of first litter born to mature mice, aged 20–27 wk before breeding
| Diet | n | Conception weight, g | Litter size | Gestation length, days | Sex ratio* | No. of male-biased litters |
|---|---|---|---|---|---|---|
| LF | 14 | 31.0 ± 4.9 | 9.2 ± 3.6 | 20.4 ± 1.5 | 0.38 | 2 |
| VHF | 11 | 41.4 ± 7.4 | 9.1 ± 3.4 | 20.4 ± 1.7 | 0.64 | 10 |
Mice (n = 16 in each group) were fed either the VHF or LF diet from 30 days of age until they had weaned their first litters of pups. The numbers under treatment are <16 and reflect either that some litters were cannibalized or the lack of receptivity of some of the mice to the males. Values for maternal weights at conception, litter size, and gestation length are means ± SD. Mothers on VHF diet were significantly heavier (P < 0.001) than ones on LF diet.
Sex ratios deviated significantly from 0.5 (P < 0.05).
The experiment summarized in Table 2 has been repeated over two breeding cycles for a second outbred strain, CF1. Again, there was no difference in the sex ratios of the pups in the two treatment groups at first litter, whereas values (0.43, LF; 0.67, VHF) deviated significantly at litter 2 (P < 0.01). It seems probable that the effect of diet is a general one in outbred strains of mice.
Discussion
That the VHF diet favors sons and the LF diet daughters is consistent with the sex allocation hypothesis of Trivers and Willard (12), in that females with the access to the greatest food resources, in this case a diet extremely high in fat, produced more sons than daughters and a predominance of male-biased litters. There are several examples where availability of extra energy to the mother seems to favor the birth of sons (8, 11, 37) and at least one example where providing additional fat had a similar outcome. Thus, American opossums in the wild, whose diets were supplemented with sardines, exhibited a higher sex ratio among their young than controls, leading the authors to conclude that consumption of excess amounts of long chain polyunsaturated fatty acids favored male offspring (38). Long chain omega-3 and -6 fatty acids have been also proposed as factors that can influence human offspring sex ratio (27). Such an explanation is unlikely for our experiments, although it is conceivable that the metabolism of large amounts of saturated fat spared the oxidation of unsaturated fatty acids.
In contrast to the outcome from the VHF diet, it was surprising that murine mothers on the LF diet, despite being adequately provisioned, delivered more daughters than sons. Food restriction of various kinds, including omission of adequate amounts of essential fatty acids, usually (16–19) but not invariably (21) leads to a low sex ratio in rodents, but this skewing is accompanied by smaller litter sizes, suggesting that male fetuses are particularly susceptible to loss. The higher vulnerability of males has also been demonstrated in rodents that are stressed in other ways during their pregnancies (22–26, 39). In the case of our experiments, mothers on the LF diet had full access to a nutritionally complete diet and were able to produce four successive litters of pups of normal size at ≈10-wk intervals, which reflects the potential of many mouse strains (40). Our results indicate that lack of adequate food is not an explanation for the higher number of female births in the LF group. It is perhaps significant that most chow-based diets, which provide amounts of fat intermediate between the VHF and LF diets tested here, seem generally to lead to the production of roughly equal numbers of males and females (29, 41). Conceivably, it is either the content of fat or the caloric density of the diet that ultimately controls which way the sex ratio becomes skewed. Because certain in-bred strains maintained on standard diets vary slightly but nevertheless significantly in the relative numbers of sons and daughters they produce (29, 41), it will be of interest to determine whether such mouse lines also differ in their responses to the LF and VHF diets and whether the skewing of sex ratio among individual mice on the diets occurs with less overlap than in the outbred strains used by us (Fig. 2).
The lack of difference in the relative numbers of male and female pups born to young mice is puzzling because the young mothers had already been exposed to the diets for 6 wk by the time they were bred (Table 2). One explanation is that several weeks of adjustment to the diets are necessary for the mice to provide a response. Another may relate to innate metabolic differences between young and mature female mice in their responses to the diets. Whatever the reason for the sex skewing, it is not a parity effect, because mature mice produced more male pups on the VHF diet and more female pups on the LF diet whether they had been bred once (Table 3) or more frequently (Table 2). Similarly, the effect is not mediated through the male, because only young stud males were used, and their only exposure to the diets occurred during the short time they were housed with the females.
Several hypotheses have been proposed to explain skewing of sex ratios in mammals where the male is always the heterogametic sex (8). These theories fall into two classes, those that operate before conception and those that favor one sex over the other after fertilization has occurred. It is feasible, for example, that conditions within the reproductive tract, such as vaginal pH (42) or the viscosity of cervical mucus (43), favor Y-sperm over X-sperm or vice versa in terms of movement to the egg or in fertilization potential. Conversely, there could be selective loss of conceptuses either before or after implantation. Such losses need not lead to smaller than usual litters, because mice generally ovulate more eggs than pups born (14, 44). The mouse model demonstrated here will allow each of these possibilities to be tested and perhaps provide a more general insight into mechanisms that operate in other species as well.
It is fascinating to consider whether these observations made on a litter-bearing species, the mouse, have any relevance to the human. Deviations in sex ratio have been observed in nonhuman primates and attributed to factors such as societal rank, competition, population density, and food availability (8, 45). Unfortunately, the group sizes have generally been small and the statistical significance of the data questionable (46, 47). Similarly, much of the information available for sex ratio deviations in humans is also confounded by a variety of unresolved variables, including sample size, although some larger demographic studies have suggested an association of increased female births with suboptimal maternal nutrition (27, 28, 48, 49). As in rodents, the shift in sex ratio in these human populations may be due the greater vulnerability of male fetuses to environmental stresses (27). However, retrospective analyses can reveal little about individual adaptations of mothers to what they consume and are unlikely to define whether particular nutrients in an otherwise adequate diet can influence whether a boy or girl is born. At present, it seems premature to extrapolate data from rodent studies to humans.
One particularly interesting cause of sex ratio variation in rodents arises from the mother's prior intrauterine position (50, 51). Females born between two males (2M) tend to assume more masculine traits, assume a dominant social role, and, when they breed, produce male-biased litters, whereas females born with no adjacent males produce litters biased toward daughters. The basis of this epigenetic phenomenon is unclear but may be caused by high androgen concentrations encountered by the 2M females while in utero. In light of the above rodent studies, it is interesting to note that women who have high testosterone levels tend to score high on dominance measure tests and conceive more sons than those women who score low on these tests (52–54). In humans, a recent positive correlation between a slightly increased fatty diet and steroid hormone concentrations in serum has been reported (55). Conceivably, there is a linkage in female mice as well as in women, between the amount of fat consumed and the production and circulating concentrations of steroid hormones. Perhaps these changes provide the basis for the sex ratio skewing observed here in mice.
Acknowledgments
We thank Dr. Kevin Fritsche for information about responses of young mice to high-fat diets, and Drs. Tom Fleming, Deborra Mullins, and Daniel Pomp for critical evaluation of the manuscript. Research was supported by U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service Competitive Research Grant 2001-35203-10693.
Abbreviations
- LF diet
diet low in saturated fat, with the majority of calories provided as sugars and complex carbohydrates
- VHF diet
diet very high in saturated fat, with most energy provided as lard
References
- 1.Hamilton W. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- 2.Nunney L, Luck R. Theor Popul Biol. 1988;33:1–30. doi: 10.1016/0040-5809(88)90002-0. [DOI] [PubMed] [Google Scholar]
- 3.Charnov E L. The Theory of Sex Allocation. Princeton: Princeton Univ. Press; 1982. [PubMed] [Google Scholar]
- 4.Nager R, Monaghan P, Griffiths R, Houston D, Dawson R. Proc Natl Acad Sci USA. 1999;96:570–573. doi: 10.1073/pnas.96.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West S, Herre E, Sheldon B. Science. 2000;290:288–290. doi: 10.1126/science.290.5490.288. [DOI] [PubMed] [Google Scholar]
- 6.West S, Sheldon B. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]
- 7.West S, Reece S, Sheldon B. Heredity. 2002;88:117–124. doi: 10.1038/sj.hdy.6800018. [DOI] [PubMed] [Google Scholar]
- 8.Clutton-Brock T H, Iason G R. Q Rev Biol. 1986;61:339–374. doi: 10.1086/415033. [DOI] [PubMed] [Google Scholar]
- 9.Flint A P F, Albon S, Jafar S. Gen Comp Endocrinol. 1997;106:374–383. doi: 10.1006/gcen.1997.6879. [DOI] [PubMed] [Google Scholar]
- 10.Kruuk L E B, Clutton-Brock T H, Albon S D, Pemberton J M, Guinness F E. Nature. 1999;399:459–461. doi: 10.1038/20917. [DOI] [PubMed] [Google Scholar]
- 11.Wauters L A, de Crombrugghe S A, Nour N, Matthysen E. Behav Ecol Sociobiol. 1995;37:189–193. [Google Scholar]
- 12.Trivers R L, Willard D E. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- 13.Labov J B, Huck U W, Vaswani P, Lisk R D. Behav Ecol Sociobiol. 1986;18:241–249. [Google Scholar]
- 14.Rugh R. The Mouse: Its Reproduction and Development. New York: Oxford Univ. Press; 1994. [Google Scholar]
- 15.Baker D E J. In: The Laboratory Rat: Reproduction and Breeding. Baker H J, Lindsay R H, Welshroth S H, editors. New York: Academic; 1979. pp. 154–168. [Google Scholar]
- 16.Meikle D, Drickamer L. J Reprod Fertil. 1986;78:587–591. doi: 10.1530/jrf.0.0780587. [DOI] [PubMed] [Google Scholar]
- 17.Wright S, Crawford C, Anderson J. Behav Ecol Sociobiol. 1988;23:357–365. [Google Scholar]
- 18.Meikle D, Thornton M. J Reprod Fertil. 1995;105:193–196. doi: 10.1530/jrf.0.1050193. [DOI] [PubMed] [Google Scholar]
- 19.Rivers J, Crawford M. Nature. 1974;252:297–298. doi: 10.1038/252297a0. [DOI] [PubMed] [Google Scholar]
- 20.Kwong W Y, Wild A E, Roberts P, Willis A C, Fleming T P. Development (Cambridge, UK) 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- 21.Zamiri M. Aust J Biol Sci. 1978;31:629–639. doi: 10.1071/bi9780629. [DOI] [PubMed] [Google Scholar]
- 22.Geiringer E. Proc Soc Exp Biol Med. 1961;106:752–754. doi: 10.3181/00379727-106-26464. [DOI] [PubMed] [Google Scholar]
- 23.Lane E A, Hyde T S. J Abnormal Psychol. 1973;82:73–80. doi: 10.1037/h0034851. [DOI] [PubMed] [Google Scholar]
- 24.Moriya A, Hiroshige T. Int J Biometeorol. 1978;22:312–315. doi: 10.1007/BF01552813. [DOI] [PubMed] [Google Scholar]
- 25.Krackow S. J Reprod Fertil. 1997;110:87–90. doi: 10.1530/jrf.0.1100087. [DOI] [PubMed] [Google Scholar]
- 26.Pratt N, Lisk R. J Reprod Fertil. 1989;87:763–769. doi: 10.1530/jrf.0.0870763. [DOI] [PubMed] [Google Scholar]
- 27.Crawford M A, Doyle W, Meadows N. Hum Reprod. 1987;2:517–520. doi: 10.1093/oxfordjournals.humrep.a136581. [DOI] [PubMed] [Google Scholar]
- 28.Andersson R, Bergstrom S. Hum Biol. 1998;70:1101–1106. [PubMed] [Google Scholar]
- 29.Schlager G, Roderick T. J Hered. 1968;59:363–365. doi: 10.1093/oxfordjournals.jhered.a107747. [DOI] [PubMed] [Google Scholar]
- 30.Drickamer L. Lab Anim Sci. 1990;40:284–288. [PubMed] [Google Scholar]
- 31.Reeves P G, Nielson F H, Fahey G C J. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 32.Kunieda T, Xian M, Kobayashi E, Imamichi E, Moriwaki K, Toyoda Y. Biol Reprod. 1992;46:692–697. doi: 10.1095/biolreprod46.4.692. [DOI] [PubMed] [Google Scholar]
- 33.SAS Institute. SAS/STAT User's Guide. Cary, NC: SAS Inst.; 1988. [Google Scholar]
- 34.Huntsberger D V, Billingsley P. Elements of Statistical Inference. Boston: Allyn and Bacon; 1977. [Google Scholar]
- 35.Littell R C, Henry P C, Ammerman C B. Anim Sci. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- 36.Wilson S, Goodale H, Kyle W, Godfrey E. J Hered. 1971;62:228–234. doi: 10.1093/oxfordjournals.jhered.a108156. [DOI] [PubMed] [Google Scholar]
- 37.Enright W, Spicer L, Kelly M, Culleton N, Prendiville D. Small Rumin Res. 2001;39:253–259. doi: 10.1016/s0921-4488(00)00199-1. [DOI] [PubMed] [Google Scholar]
- 38.Austad S, Sunquist M. Nature. 1986;324:58–60. [Google Scholar]
- 39.Pratt N, Lisk R. Behav Neural Biol. 1990;54:1–12. doi: 10.1016/0163-1047(90)91201-l. [DOI] [PubMed] [Google Scholar]
- 40.Silver L. Mouse Genetics: Concepts and Applications. New York: Oxford Univ. Press; 1995. [Google Scholar]
- 41.Howard A, McLaren A, Michie D, Sander G. J Genet. 1954;53:200–214. [Google Scholar]
- 42.Pratt N, Huck U, Lisk R. Behav Neural Biol. 1987;48:310–316. doi: 10.1016/s0163-1047(87)90864-8. [DOI] [PubMed] [Google Scholar]
- 43.Martin J. Hum Reprod. 1997;12:611–616. doi: 10.1093/humrep/12.3.611. [DOI] [PubMed] [Google Scholar]
- 44.Ishakawa H, Endo A. J Reprod Fertil. 1996;108:167–170. doi: 10.1530/jrf.0.1080167. [DOI] [PubMed] [Google Scholar]
- 45.Hiraiwa-Hasegawa M. Trends Ecol Evol. 1993;8:395–399. doi: 10.1016/0169-5347(93)90040-V. [DOI] [PubMed] [Google Scholar]
- 46.Packer C, Collins D, Eberly L. Philos Trans R Soc London. 2000;355:1627–1635. doi: 10.1098/rstb.2000.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown G, Silk J. Proc Natl Acad Sci USA. 2002;99:11252–11255. doi: 10.1073/pnas.162360599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jongbloet P H, Zielhuis G A, Groenewoud H M M, Pasker-de Jong P C M. Environ Health Perspect. 2001;109:749–752. doi: 10.1289/ehp.01109749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koziel S, Ulijaszek S J. Am J Phys Anthropol. 2001;115:71–79. doi: 10.1002/ajpa.1058. [DOI] [PubMed] [Google Scholar]
- 50.Vandenbergh J, Huggett C. Proc Natl Acad Sci USA. 1995;91:1155–1159. [Google Scholar]
- 51.vom Saal F, Clark M, Galef B, Drickamer L, Vandenbergh J. In: Encyclopedia of Reproduction. Neill J D, Knobil E, editors. Vol. 2. New York: Academic; 1999. pp. 893–900. [Google Scholar]
- 52.Grant V. Br J Med Psychol. 1994;67:343–351. doi: 10.1111/j.2044-8341.1994.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 53.Grant V. Hum Reprod. 1996;11:2371–2375. doi: 10.1093/oxfordjournals.humrep.a019117. [DOI] [PubMed] [Google Scholar]
- 54.Grant V, France J. Biol Psychol. 2001;58:41–47. doi: 10.1016/s0301-0511(01)00100-4. [DOI] [PubMed] [Google Scholar]
- 55.Dorgan J, Hunsberger S, McMahon R, Kwiterovich P J, Lauer R, Van Horn L, Lasser N L, Stevens V J, Friedman L A, Yanovski J A, et al. J Natl Cancer Inst. 2003;95:132–141. doi: 10.1093/jnci/95.2.132. [DOI] [PubMed] [Google Scholar]