Abstract
Stem cells are thought to reside in regulatory microenvironments (“niches”) generated by stable stromal neighbors. To investigate the significance of empty niches vacated by stem cell loss, we studied Drosophila ovarioles, which maintain two to three germ-line stem cells in a niche requiring adhesive stromal cap cells and Decapentaplegic signals. After experimentally emptying the germ-line stem cell niche, cap cell activity persists for several weeks. Initially, somatic inner germarium sheath cells enter the empty niche, respond to Dpp, but fail to divide. Subsequently, follicle cell progenitors, including somatic stem cells enter the niche, respond to Dpp, and proliferate as long as cap cells remain. Proliferation requires the normal hedgehog signal of the somatic stem cells as well as proximity to the niche. Thus, empty niches can persist, signal incoming cells, and support ectopic proliferation. Similar events may underlie some disease states.
Specific microenvironments known as niches are thought to regulate many stem cells types by rationing growth and differentiation factors (1, 2). Within a niche, a lost stem cell can be replenished when a remaining stem cell is caused to divide symmetrically (3). Moreover, niches can be colonized after the transplantation of isolated stem cells (4) or the immigration of cells from other niches (5, 6). The ability of niches to dynamically modulate cellular behavior suggests that they may play important roles in cellular transdifferentiation (7–9) and in proliferative disorders (10). Exogenous cells may contact endogenous niches after normal stem cell turnover, tissue damage, cellular invasion, or after experimental cell transfers. Consequently, it is important to understand what happens when cells and niches interact outside their normal developmental routines.
The well studied germ-line stem cell (GSC) niche within the germarium region (Fig. 1a) of the Drosophila melanogaster ovariole provides an excellent opportunity to address these issues (11, 12). Each niche supports two to three GSCs and regulates their asymmetric division to generate a daughter stem cell as well as a daughter cystoblast (CB) that founds a new follicle. Unlike GSCs, CBs express the bag of marbles (bam) gene and both possess a vesicle-rich organelle known as the fusome whose shape distinguishes early germ cells. Several distinct somatic cell types including terminal filament, cap cells (CpCs), and inner-germarium sheath cells (IGSs) reside near the niche (Fig. 1 a and b). CpCs play a crucial role by associating tightly with GSCs (13) and have been proposed to provide the Decapentaplegic (Dpp) signals that maintain GSCs in the niche and control their division (14). Terminal filament cells prefigure ovariole development and may also mediate niche signals in adults (15). IGSs interact primarily with differentiating stem cell daughters and vary in number as adults age (16). Some distance from the niche proper lie two to three somatic stem cells (SSCs) that give rise to ovarian follicle cells (Fig. 1a) (16). SSCs, unlike GSCs, proliferate in response to hedgehog (hh) signaling (17, 18), and the division of these two stem cell populations is not precisely coordinated (16), implying that SSCs lie outside the GSC niche.
Figure 1.
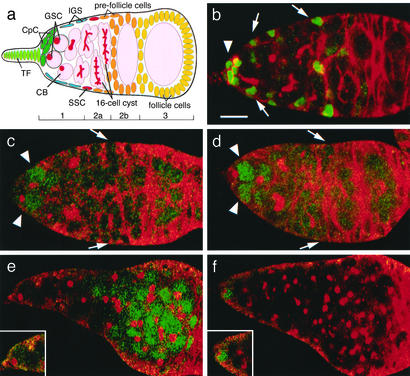
The GSC niche is associated with local enhanced Dpp signal transduction. (a) A schematic drawing of a Drosophila germarium showing germ-line cells (pink) including the GSC, CB, and developing cysts (note branched fusomes, red). Somatic cells are terminal filament (TF; yellow-green), CpCs (green), IGSs (blue), and prefollicle cells (yellow). (b) A germarium from strain PZ1444-lacZ, an enhancer trap line that marks CpCs (strong green, arrowhead) and IGSs (light green, arrow). Hts staining is shown in red to mark fusomes and cell boundaries. (c) A wild-type germarium stained for pMAD (green) and Hts (red). High levels of pMAD are present only in GSCs (arrowhead). Elevated staining is not observed near the SSCs (arrows). (d) A Dad-lacZ germarium stained for LacZ (green) and Hts (red). LacZ is expressed strongly only in GSCs (arrowhead) and weakly in young CBs. Elevated staining is not seen near the SSCs (arrows). (e and f) Tumorous germaria from a c587-GAL4/UAS-dpp strain (e, see Materials and Methods) or a bam strain (f) stained for pMAD (green) and Hts (red). pMAD is widely distributed in the pattern dictated by the c587 line, indicating the early germ cells are competent to respond to Dpp throughout the germarium. In the bam tumor, pMAD is found only in endogenous GSCs located at the anterior tip, indicating that this location is unique in its ability to up-regulate the Dpp pathway. Insets in e and f show a GSC at higher magnification. (Bar = 10 μm.)
Materials and Methods
Drosophila Stocks.
y ry was used as wild type. The following fly stocks used in this study were described either in FlyBase or as otherwise specified: dpphr4, dpphr56, hhts2, hh-lacZ, bamΔ86/TM3, P[w+; hsp70-bam+]18d, and P[w+; hsp70-bam+]11d. The enhancer trap lines PZ0078 (see figure 4C in ref. 19), PZ1444 (16), PZ2954 (16), PZ10613, and Dad-lacZ were isolated as new insertions of the PZ transposon (20). The PZ element in Dad-lacZ was localized within the 5′ UTR of Dad (T.K. and A.S., unpublished data). The PZ10613 insertion was mapped by sequencing flanking DNA just upstream of patched (A. Mahowald and A.S., unpublished data). The c587 GAL4-expressing line (21) was found to drive UAS-lacZ expression in region 2 somatic cells in the germarium (D. Drummond-Barbosa and A.S., unpublished data). When used to express UAS-dpp, this driver leads to an exceptionally large expansion in the number of GSCs (T. Xie and A.S., unpublished data).
Ablation of GSCs.
hs-Bam flies were crossed to PZ1444, PZ2954, hh-LacZ, or Dad-lacZ, and progeny were heat-shocked as follows to ablate GSCs: 37°C for 1 h, 25°C for 1 h, and 37°C for 1 h. Temperature-sensitive mutants of dpp and hh flies (hs-Bam; dpphr4/dpphr56 and hs-Bam; hhts2) were cultured at 18°C. To ablate GSCs, they were heat-shocked as above, held at 18°C for 5 days, and finally transferred to 29°C. BrdUrd incorporation was examined 5 days after shifting to 29°C (10 days after GSC ablation).
Generating Clones.
Mitotic clones were generated according to Margolis and Spradling (16). Three-day-old X-15–29/X-15–33; MRKS, hs-flp/hs-Bam flies were heat-shocked as described previously and kept at 25°C. Clones were identified by anti-LacZ staining.
Immunostaining and Fluorescence Microcopy.
Ovaries were dissected in 1× Grace's medium (BioWhittaker) and fixed for 10–20 min in fixation solution, which is 2 vol of 1× Grace's medium and 1 vol of 16% EM-grade formaldehyde (Ted Pella Inc., Redding, CA). After several washes with PBX (10 mM NaH2PO4/NaHPO4, pH 7.4/175 mM NaCl/0.2% Triton X-100), the samples were preabsorbed for 30 min or longer with PBX containing 5% of normal goat serum (The Jackson Laboratory). Ovaries were rinsed once with PBX and then incubated for 4 h at room temperature or overnight at 4°C in primary Ab diluted with PBX containing 0.5% BSA. After several washes with PBX, ovaries were incubated with secondary Ab diluted with PBX containing 0.5% BSA for at least 2 h at room temperature or overnight at 4°C. After several more washes with PBX, ovaries were stained with 4′,6-diamidino-2-phenylindole (DAPI), rinsed with PBX without Triton, and mounted with Vectashield (Vector Laboratories). Confocal images were taken by using a Leica TCS-NT microscope.
The following antisera were used: anti-antiphosphorylated Mad (pMAD; PS1) a gift from Peter ten Dijke, Ludwig Institute, Uppsala (1:200); anti-β-galactosidase (1:1,000) (Cappel); mAb 1B1 (anti-Hts) from Hybridoma Bank, Iowa City (1:50); mouse monoclonal anti-BrdUrd (1:50; Becton Dickinson), and rat monoclonal anti-BrdUrd (1:100; Abcam, Cambridge, U.K.). Secondary Abs were goat anti-mouse, goat anti-rat, or goat anti-rabbit IgG conjugated to Alexa 488 or Alexa 568 (1:400; Molecular Probes).
ApopTag Staining.
We used an ApopTag in situ apoptosis detection kit (Serologicals, Clarkston, GA) to detect IGS cell death in agametic germaria. Ovaries from PZ1444; hs-bam flies 4 days after heat shock were dissected and fixed as described, and washed with PBX for 30 min. Ovaries were then washed twice for 5 min and incubated for 1 h at 37°C according to the manufacturer's protocol. Reactions were stopped and ovaries were rinsed with PBX and immunostained as described previously.
BrdUrd Labeling in Vivo.
Ovaries were dissected in 1× Grace's medium, transferred into an Eppendorf tube, and incubated in 1× Grace's medium containing 20 μM BrdUrd (Sigma) for 1 h at 25°C. Ovaries were washed twice with 1× Grace's medium and fixed as described previously. DNA was denatured in 2 M HCl for 30 min, neutralized with 100 mM borax for 2 min, and stained with anti-BrdUrd Abs. For double-staining, BrdUrd-labeled and fixed ovaries were first incubated with another primary Ab, then fixed again in Grace's medium containing 8% of formaldehyde for 20 min. After several washes with PBX, DNA was denatured, neutralized, and ovaries were stained with anti-BrdUrd Ab and with appropriate secondary Abs as described previously.
Results
Mapping the GSC Niche.
Previous studies did not adequately define the precise size and location of the GSC niche. Because GSCs require a Dpp signal to maintain their stem cell identity, we reasoned that the niche can be no larger that the region capable of stimulating a competent germ cell to activate its Dpp pathway. Immunostaining of wild-type ovarioles with pMAD (22) revealed that only GSCs and possibly CBs activate the Dpp pathway at the highest levels (Fig. 1c, arrowhead). This conclusion was supported further by experiments showing that the Dpp target gene Dad (23), assayed with a Dad-lacZ enhancer trap, is also strongly up-regulated only in these same cells (Fig. 1d, arrowhead). Neither reporter of Dpp signal reception is highly activated in the vicinity of the SSCs (arrows). These results argue that the GSC niche is limited to the very anterior of the germarium adjacent to CpCs.
It remains possible that the GSC niche is actually larger than suggested by the previous experiment. Increasing germ-cell differentiation away from the anterior tip in a wild-type ovariole rather than small niche size might have prevented a more widespread Dpp response. To address this question, we first showed that competent germ cells can respond far from their normal location when Dpp signaling is expanded. By using a GAL4 driver we expressed Dpp in region 2 somatic cells. Under these conditions, germ cells throughout the germarium remain as GSCs and strongly express pMAD in a pattern determined by the driver line (Fig. 1e). To learn how a similar “test” population of early germ cells responds in a germarium where Dpp signaling has not been elevated, we examined bam mutant files, whose germ cells are arrested before CB differentiation (24). Under these conditions high levels of pMAD are detected only in a small number of germ cells associated with CpCs, as in wild type (Fig. 1f). These experiments argue that the normal GSC niche is restricted to a small region surrounding the CpCs. To be affected by this niche, we would expect that ectopic cells would have to enter this region.
Empty Niches Are Quasi-Stable.
To determine the stability and activity of an empty GSC niche we forced the resident GSCs to differentiate and exit the niche by briefly expressing Bam protein ectopically by using a heat-shock-Bam transgene (hs-bam) (ref. 24; Fig. 2). After a single heat shock, GSCs differentiate and exit the niche within 24 h. After 4 days, the youngest germ cells are in region 2b cysts (Fig. 2a), whereas after 7 days, the only germ cells remaining in the germarium are in a single stage-1 follicle (Fig. 2b). We analyzed the cellular composition surrounding the niche at various time after GSC departure by using several different criteria. Terminal filament cells were recognized by morphology and hh expression. CpCs were identified by hh expression, because they are strongly labeled by the PZ0078 enhancer trap (ref. 19; see Materials and Methods) and by PZ1444 (16). IGSs were specifically labeled with PZ1444 and the patched enhancer trap PZ10613. Follicle cell progenitors were labeled by using two specific markers: the c587 GAL4 line driving UAS-lacZ (see Materials and Methods) and PZ2954 (16). Consistent results were obtained with all these markers.
Figure 2.
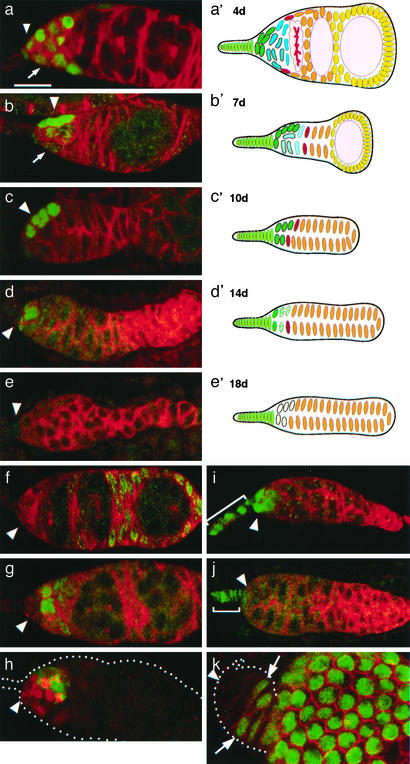
Empty GSC niches interact with two successive populations of ectopic cells. The changing cellular composition of the GSC niche and surroundings is shown 4 (a, a′), 7 (b, b′), 10 (c, c′),14 (d, d′), and 18 (e, e′) days after the ablation of GSCs by using hs-bam. Micrographs (a–e) of PZ1444 germaria are shown (anterior is left) stained with anti-β-galactosidase (green) to label CpCs (arrowhead) and IGSs (arrow), and anti-fusome Abs (red) against Hts protein. (f) Enhancer trap line PZ2954 (green) shows the position of follicle cell progenitors 4 days after GSC ablation. Hts staining is shown in red. (g) Enhancer trap line PZ10613 (green) shows the position of IGSs adjacent to the CpCs (unlabeled, arrowhead) 4 days after ablation. Hts staining is shown in red. (h) Staining for ApopTag (green) 4 days after ablation reveals that IGSs die by programmed cell death (PZ1444-lacZ is shown in red). The stability of terminal filament cells (bracket) is revealed by using hh-lacZ at 10 (i) and 18 days (j). CpCs are also stable (a–d and i) but disappear by 18 days as shown by staining for PZ1444 (e) and hh-lacZ (j). (k) Lineage analysis of follicle cell behavior after GSC ablation. An SSC clone generated by inducing FLP-mediated recombination at the same time as GSC ablation is shown after staining 7 days later. Follicle progenitor cells (green) lie posterior to an unlabeled group (arrowhead) consisting of CpCs and four to five IGSs. (a′–e′) The changing shape and cellular composition of the germarium after GSC ablation is summarized diagrammatically. The locations of CpCs are indicated by an arrowhead. (Bar = 10 μm.)
We found that terminal filament cells and CpCs, the somatic cells comprising the niche, remain stable for an extended period after GSCs depart. Terminal filament cells change little for at least 3 weeks as revealed by staining with hh-lacZ (Fig. 2 i and j, bracket). Moreover, CpCs, the key niche component, appeared normal even when all germ cells were gone 8–10 days after heat shock, and continued to express normal gene markers for an additional week (Fig. 2 b–d and i, arrowheads). However, by 18 days after GSC loss, CpCs could no longer be detected with either PZ1444 (Fig. 2e), hh (Fig. 2j), or PZ0078 (data not shown). Thus, the key somatic cells of the niche remain stable for several weeks after the loss of its stem cells.
Ectopic Somatic Cells Enter the Vacated Niche.
In contrast, IGSs were much less stable than CpCs after the loss of early germ cells. The number of cells expressing the IGS markers fell rapidly after heat shock (Fig. 2 a, b, and g) and no IGSs could be detected beginning 8–9 days after ablation (Fig. 2c, data not shown). The loss of IGS gene expression has been described in agametic germaria produced during development (16) or after GSC differentiation (3). Staining with ApopTag revealed that IGSs undergo apoptosis as germ cells leave the anterior region of the germarium (Fig. 2h). Hence, IGSs turn over in the absence of the early germ cells with which they normally interact.
The departure of germ cells and death of IGSs brings the SSCs and daughter prefollicle cells increasingly closer to the GSC niche. We documented this in two ways. First, all of the somatic cells not staining with terminal filament, CpC, or IGS markers were labeled by prefollicle cell enhancer traps (Fig. 2f). These cells include the SSCs and their young progeny, which have not yet become incorporated into a follicular layer. To assess the location of the SSCs after GSC differentiation, we induced somatic cell clones at the same time as hs-bam expression. Four or more days later, after transient clones have departed the germarium, only ovarioles with a marked SSC clone will extensively label prefollicle cells in the germarium. The SSCs at the anterior margins of such clones (Fig. 2k, arrows) moved toward the CpCs (Fig. 2k, arrowhead) as IGSs were lost. These changes in cell organization after GSC departure are summarized pictorially in Fig. 2 a′–e′ and numerically in Fig. 3.
Figure 3.
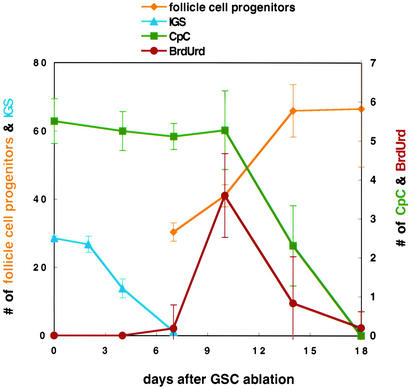
Somatic cell changes in the vicinity of the GSC niche after stem cell departure. A graph summarizes changes in the number of follicle cell progenitors (yellow), IGSs (blue), CpCs (green), and the number of somatic cells in the vacated GSC niche that label with BrdUrd (red) as a function of time after ablation of the GSC by a heat-induced pulse of Bam protein. Note that two different scales are used. Data are from the experiments of Figs. 2 and 4.
Ectopic Niche Cells Activate Dpp Signaling.
These experiments show that two distinct somatic cell populations, IGSs and follicle cell progenitors, sequentially enter the empty GSC niche after the differentiation and departure of GSCs. To determine how the incoming cells respond to the local Dpp signal of the niche, we looked for induced expression of the Dad-lacZ transgene. Within 4 days of GSC departure (Fig. 4a), strong expression of Dad-lacZ is observed in three to four IGSs (arrow) located next to the CpCs (arrowhead). These niche-occupying IGSs continue to express Dad-lacZ until they turn over. Subsequently, those incoming follicle cell progenitors that come to lie near the CpCs (Fig. 4b, arrow) express Dad-lacZ up until the time CpCs shut down (Fig. 4c). These studies indicate that both types of ectopic somatic cell respond to a major niche signal when they come to occupy the empty GSC niche.
Figure 4.
The niche induces ectopic cells to up-regulate Dpp signaling and to divide. (a–c) Cells responding to Dpp signaling were assayed by using Dad-lacZ. Germaria 4 (a), 10 (b), and 18 (c) days after GSC ablation are shown. Staining is with anti-β-galactosidase (green) and anti-Hts (red); arrowhead indicates CpCs. (d–f) To determine whether cells entered the cell cycle, BrdUrd incorporation was assayed in PZ1444-lacZ females. Germaria were stained for LacZ (red) and BrdUrd (green) at various times after GSC ablation. (d) Seven days, ectopic IGSs in the niche are not labeled; only cells associated with developing follicles have incorporated BrdUrd; SSCs are also negative at this time (arrow). (e) Ten days, ectopic follicle cell progenitors located in the niche adjacent to CpCs (arrowhead) strongly incorporate BrdUrd. (f) Eighteen days, no incorporation is seen after CpC function (arrowhead) is lost. The germarium is outlined. (Bar = 10 μm.)
Ectopic Follicle Cell Progenitors but Not IGSs Divide Within the Vacated Niche.
We next examined BrdUrd incorporation and did cell counts to determine whether the ectopic cells associated with the niche are stimulated to cycle and divide. During the first 7 days after GSC departure, BrdUrd is incorporated only in the growing prefollicle cells and germ cells that will give rise to egg chambers but not in somatic cells close to the niche (Fig. 4d). After 4 days, no BrdUrd is observed in the vicinity of the SSCs (Fig. 4d, arrow); instead, only daughter somatic cells continue to label. Thus, despite up-regulating Dpp signal reception after occupying the vacant niche, IGSs are not stimulated to enter the cell cycle.
However, the follicle cell progenitors that enter the niche 7 days after GSC ablation respond differently. These cells begin to incorporate BrdUrd after coming in contact with the CpCs (Fig. 4e). The number of BrdUrd-labeled cells reaches a maximum at 10 days (Fig. 3), and falls gradually to 0 by 18 days (Fig. 4f). During this time the number of prefollicle cells increases >2-fold from 30 to >60 cells (Fig. 3), and as a result the anterior germarium becomes much longer (Fig. 2 b vs. e). Because only two to four cells (mean = 3) adjacent to the CpCs are cycling (Fig. 3), they would need to undergo ≈10 rounds of division to account for this increase, about one per day.
Ectopic Cell Division Still Requires hedgehog.
Ectopic follicle cell progenitors that enter the niche after IGSs regress up-regulate Dpp signaling and enter the cell cycle. We next investigated the functional role of Dpp and the Hh signal normally used by follicle cell progenitors in this proliferation. dppts or hhts flies that also carry hs-bam were cultured at the permissive temperature. To initiate GSC loss they were subjected to a brief heat shock, returned to the permissive temperature for 5 days, and then shifted to restrictive temperature (29°C) to reduce niche signaling before the arrival of the follicle cell progenitors. The behavior of follicle cell progenitors was followed beginning 1 week after heat shock. One unexpected outcome of this protocol was that a higher number of follicle cell progenitors near the niche became BrdUrd-labeled at 29°C than at 22°C (Fig. 5a vs. Fig. 4e), suggesting that niche size may expand slightly with temperature.
Figure 5.
Ectopic niche cells still require hedgehog. Control flies (PZ1444) and flies containing hs-bam and either a temperature-sensitive allele of dpp (a) or hh (b) that had been grown at the permissive temperature were subjected to a brief heat shock, incubated for 5 more days at the permissive temperature, and then shifted to the nonpermissive temperature for 5 days. BrdUrd incorporation within ectopic follicle progenitor cells in the vacated niche was observed in control flies (see Fig. 4e) and when Dpp was reduced (a) but not when Hh was reduced (b). The number of BrdUrd-incorporating cells was counted, and the average values in control (PZ1444) and the experimental genotypes were plotted (c). (d) The effects of ectopic Hh during niche repopulation was determined by measuring BrdUrd incorporation in flies containing both hs-hh and hs-bam transgenes, and that were repeatedly subjected to heat shocks for 18 days before analysis. Under these conditions, BrdUrd incorporation is observed in follicle cell progenitors throughout much of the germarium, not just in the niche. (e) A model showing the stimulation of ectopic SSCs (red) by Hh and possibly by Dpp in the vacated GSC niche. (Bar = 10 μm.)
The results of this experiment were unexpected. Follicle cell progenitors in dppts flies at the restrictive temperature incorporated BrdUrd with the same frequency as controls (Fig. 5 a and c). In contrast, when Hedgehog signaling is impaired by using hhts, the niche is no longer able to induce nearby follicle cell progenitors to incorporate BrdUrd (Fig. 5 b and c). Thus, follicle cell progenitors in the niche continue to require their normal Hh signal to proliferate, despite having up-regulated Dpp signaling. To investigate further the role of this signal, we ectopically expressed Hh after GSC ablation by using an hs-hh transgene. This treatment greatly increased the number of BrdUrd-incorporating follicle cell progenitors and now allowed follicle cell progenitors remaining outside the niche to cycle (Fig. 5d). These results argue that after GSC loss, follicle cell progenitors proliferate in the niche, because it provides the only effective Hh source (Fig. 2 i and j). However, these studies do not rule out a role for Dpp, because the dppts strain we tested may not reduce the level of Dpp signaling sufficiently in this tissue to affect proliferation.
Discussion
Niches Are Small and Stable.
These experiments provide new insight into the structure and behavior of GSC niches. We showed that GSC niches are small and confined to the region near the CpCs at the anterior of the germarium. In the absence of their normal stem cells, GSC niches remain functional for nearly 3 weeks, a significant fraction of the Drosophila adult lifetime. During the first 2 weeks there is little change in either niche structure or activity as measured by the ability to promote Dpp signaling in neighboring cells. The number and appearance of terminal filament and CpCs, the two cell types implicated in niche function (2), remain constant, despite changes in neighboring cells. Only after 2 weeks do CpCs, and the ability of the niche to support cell division, start to decline.
The robust nature of the GSC niche revealed here is likely to explain several previous observations. Morphologically normal ovariole tips containing cap and terminal filament cells (16) are known to form in the complete absence of germ cells (25–27). Somatic cells proliferate in such agametic germaria (27), and the growing cells express follicle cell markers (16). Our experiments suggest that the stability of the GSC niches in such germaria is likely to be a critical factor determining the nature and extent of this proliferation.
Some but Not All Ectopic Cells Divide After Entering the Empty GSC Niche.
We found that although all ectopic cells up-regulated Dpp signaling after entering the niche, their response depended on their differentiated state. IGSs do not incorporate BrdUrd or divide and soon undergo apoptosis. In contrast, ectopic follicle cell progenitors are able to undergo many rounds of division in a foreign niche. Because neither markers nor lineage studies can unambiguously distinguish SSCs from their immediate prefollicle cell progeny, it remains possible that only SSC can proliferate in the empty niche. However, the number of ectopic BrdUrd-incorporating cells within the niche (three to four at 22°C, seven to nine at 29°C) was somewhat larger than the expected number of SSCs, suggesting that both SSCs and early prefollicle cells are able to respond. Regardless, our results show that the reprogramming ability of an empty GSC niche seems to be quite limited. The fate of the incoming cells was not changed, and only follicle cell progenitors but not IGSs were stimulated to divide. Thus, the growth-promoting action of a particular stem cell niche is limited and requires a matched companion cell to result in stem cell activity.
The failure of IGSs to divide in response to niche signals is probably not because these cells are unable to proliferate. IGSs are probably capable of cycling because their number varies with age (3) and they are sporadically labeled in lineage tracing experiments (16, 28). IGSs probably do not respond to niche signals like follicle cell progenitors because they additionally require maintenance signals normally provided by developing cysts (3). Nonetheless, the niche may influence these cells, because we observed that distal IGSs closest to the departing germ cells were the first to enter apoptosis (Fig. 2h). Proximity to the niche may prolong the survival of proximal IGSs but may be insufficient to permanently reverse apoptotic stimuli.
Nature of the Niche Requirement.
Several observations showed that niche cells play an essential positive role in stimulating the renewed division of follicle cell progenitor cells. These cells originally cease dividing after the last cyst moves by and only begin to divide again after entering the vacated GSC niche several days later. Although all SSC progeny proliferate during normal development, after germ-cell departure only follicle cell progenitors that closely approach and/or contact a CpC ever incorporate BrdUrd and undergo mitosis. Dpp reception as indicated by Dad-lacZ expression within follicle cell progenitors correlates closely with this cell division but surprisingly, we were unable to demonstrate a role for this major niche signal.
Our incomplete knowledge of how the division of SSCs and follicle cell progenitors is normally regulated limits our ability to interpret these observations. Follicle cell progenitors in their normal location respond to Hh; however, it remains unclear whether there is a local source of this signal or whether it is supplied by the CpCs, which are located four to five cells away. IGSs adjacent to the SSCs form essential adherens junctions with SSCs (28) and may directly provide all of the Hh signal SSCs require. This latter model makes the behavior of follicle cell progenitors after GSC loss easier to explain.
We documented that Hh levels continue to play a critical role in regulating the proliferation of follicle cell progenitors after GSC loss. After cysts depart, IGSs lose their maintenance signal and probably stop providing Hh to follicle cell progenitors. Only when these cells find a new source of Hh within the niche are they able to divide again. This finding is consistent with our observation that ectopic division within the empty niche is blocked when its hh activity is experimentally reduced, whereas greatly increasing hh activity causes follicle cell progenitors to divide throughout the germarium independently of proximity to the niche. Thus, cells which have lost their growth signals due to changes in tissue architecture likely become dependent for further proliferation on the availability of substitute signals in their vicinity.
Empty Niches May Be Biologically Significant.
Regardless of the exact mechanism, the observation that an empty GSC niche stimulates susceptible ectopic cells to divide expands our knowledge of niche biology. Our results suggest that it will be important to determine the number, location, and biological properties of all of the stem cell niches within adult tissues. After loss of their normal stem cells, these niches will likely retain the ability for some finite period to stimulate the proliferation of particular subsets of cells. Susceptible cells may include foreign stem cells (such as SSCs) that require a signal present in the niche, as well as a range of other cells. Because we found that niches can up-regulate a signaling pathway (e.g., Dpp) within incoming cells, empty niches have the potential to alter the cell fate as well as the growth properties of at least some susceptible and/or incompletely differentiated cells. We found no evidence of such reprogramming in these studies, however.
The biological properties of empty tissue niches may be of medical importance as well. The presence of a substantial number of empty niches might make a tissue a favorable substrate for cell therapy, or conversely put it at risk for supporting an early tumor metastasis. Perhaps most directly, our results suggest that empty niches play a role in benign cell growths, the condition which most resembles the cell accumulations within ovariolar tips lacking germ cells. Such growths commonly occur in many epithelial cell tissues and increase in frequency with age. Our findings suggest that growth stimulation by vacated stem cell niches may contribute to cell dysplasia.
Acknowledgments
We thank Dr. Peter ten Dijke for providing pMAD antiserum and Dr. D. McKearin (University of Texas Southwestern Medical Center, Dallas) for sending the hs-bam flies.
Abbreviations
- Dpp
Decapentaplegic
- GSC
germ-line stem cell
- SSC
somatic stem cell
- IGS
inner-germarium sheath cell
- pMAD
antiphosphorylated Mad
- CB
cystoblast
- CpC
cap cell
References
- 1.Watt F M, Hogan B L. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 2.Spradling A, Drummond-Barbosa D, Kai T. Nature. 2001;414:14–18. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 3.Xie T, Spradling A C. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 4.Brinster R L, Zimmermann J W. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimura E K, Jordan S A, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson I J, Barrandon Y, Miyachi Y, Nishikawa S. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 6.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 7.Gussoni E, Soneoka Y, Strickland C D, Buzney E A, Khan M K, Flint A F, Kunkel L M, Mulligan R C. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 8.Brazelton T R, Rossi F M V, Keshet V I, Blau H M. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 9.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman I L, Grompe M. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 10.Kim K M, Shibata D. Oncogene. 2002;21:5441–5449. doi: 10.1038/sj.onc.1205604. [DOI] [PubMed] [Google Scholar]
- 11.King R S. Ovarian Development in Drosophila melanogaster. New York: Academic; 1970. [Google Scholar]
- 12.Xie T, Spradling A C. In: Stem Cells. Marshak D, Gardner R, Gottlieb D, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 2001. pp. 129–148. [Google Scholar]
- 13.Song X, Zhu C H, Doan C, Xie T. Science. 2002;296:1855–1858. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- 14.Xie T, Spradling A C. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 15.King F J, Szakmary A, Cox D N, Lin H. Mol Cell. 2001;7:497–508. doi: 10.1016/s1097-2765(01)00197-6. [DOI] [PubMed] [Google Scholar]
- 16.Margolis M, Spradling A. Development (Cambridge, UK) 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- 17.Forbes A J, Lin H, Ingham P W, Spradling A C. Development (Cambridge, UK) 1996;122:1125–1135. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Kalderon D. Nature. 2001;410:599–604. doi: 10.1038/35069099. [DOI] [PubMed] [Google Scholar]
- 19.Spradling A C. In: The Development of Drosophila melanogaster. Bate M, Martizen-Arias A, editors. Plainview, NY: Cold Spring Lab. Harbor Press; 1993. pp. 1–70. [Google Scholar]
- 20.Karpen G H, Spradling A C. Genetics. 1992;132:737–753. doi: 10.1093/genetics/132.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manseau L, Aradaran A, Brower D, Budhu A, Elefant F, Phan H, Philp A V, Yang M, Glover D, Kaiser K, et al. Dev Dyn. 1997;209:310–322. doi: 10.1002/(SICI)1097-0177(199707)209:3<310::AID-AJA6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Tanimoto H, Itoh S, ten Dijke P, Tabata T. Mol Cell. 2002;5:59–71. doi: 10.1016/s1097-2765(00)80403-7. [DOI] [PubMed] [Google Scholar]
- 23.Tsuneizumi K, Nakayama T, Kamoshida Y, Kornberg T B, Christian J L, Tabata T. Nature. 1997;389:627–631. doi: 10.1038/39362. [DOI] [PubMed] [Google Scholar]
- 24.Ohlstein B, McKearin D. Development (Cambridge, UK) 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- 25.Fielding C J. J Embryol Exp Morphol. 1967;17:375–384. [Google Scholar]
- 26.Niki Y, Okada M. Roux's Arch Dev Biol. 1981;190:1–10. doi: 10.1007/BF00868697. [DOI] [PubMed] [Google Scholar]
- 27.Engstrom L, Caulton J H, Underwood E M, Mahowald A P. Dev Biol. 1982;91:163–170. doi: 10.1016/0012-1606(82)90019-7. [DOI] [PubMed] [Google Scholar]
- 28.Song X, Xie T. Proc Natl Acad Sci USA. 2003;99:14813–14818. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]