Abstract
The mechanism of speciation is a central problem in evolutionary biology. In free-spawning animals with no complex mating behavior, prezygotic reproductive isolation (speciation) could result from the rapid divergence of genes coding for sperm and egg proteins that bind each other during fertilization. In abalone, sperm lysin evolves rapidly by positive Darwinian selection. The egg vitelline envelope receptor for lysin had previously been shown to evolve neutrally and be subjected to concerted evolution. Several mathematical simulations predict that both male and female reproductive proteins should evolve rapidly by positive selection. Here we report that the sequence diversity of the amino-terminal end of the egg vitelline envelope receptor for lysin has been promoted by positive Darwinian selection. These data provide molecular support for theoretical models showing that the two sexes are locked in a “coevolutionary chase” that could be driven by processes such as sexual selection, sexual conflict, or microbial attack (pathogen avoidance). The result of this continuous coevolution of the gamete recognition system could be the splitting of one population into two that are reproductively isolated (speciation).
Keywords: speciation‖sexual conflict‖sexual selection‖fertilization‖ sperm, egg interaction
Animal species that broadcast gametes into water rely on sperm–egg surface recognition events to mediate fertilization. In many congeneric marine invertebrates, fertilization is species specific, meaning that homospecific mixtures of gametes form zygotes more efficiently than do heterospecific mixtures. In free-spawning species, the binding of surface proteins on sperm and egg is the underlying molecular basis for species-specific fertilization and, potentially, prezygotic reproductive isolation. The rapid evolutionary divergence of gamete surface proteins has been documented in protistans, fungi, plants, and animals and could be part of the speciation process (1). This rapid evolution in gamete recognition systems is often accompanied by positive Darwinian selection, indicating there is positive adaptive value in altering the primary structure of the protein (2).
Abalone are free-spawning marine archeogastropod mollusks, seven species of which inhabit the coast of California. Some of these species have overlapping breeding seasons and habitats; yet despite the potential for hybridization, each species remains distinct. Abalone eggs are enclosed in an elevated vitelline envelope (VE) made of glycoproteinaceous fibers that are held together by interfiber hydrogen bonds. Abalone sperm bind to the VE and release from their acrosome the 16-kDa protein lysin. Lysin binds to the VE fibers and nonenzymatically causes them to lose cohesion to each other, unraveling the compact structure of the VE and creating a hole through which the sperm swims to reach the egg cell membrane (3). Lysin is species specific at dissolving VEs and this specificity can be attributed to at least 23 residues that evolve rapidly by positive selection (4, 5).
Lysin binds species-specifically with high affinity (Kd ≈ 10−9 M) to VERL (VE receptor for lysin), a major component of the VE. VERL is a giant rod-like, ≈1,000-kDa glycoprotein composed of roughly equal amounts of protein and carbohydrate (6). The full-length sequence of VERL from the red abalone (Haliotis rufescens) is 3,722 amino acid residues, 3,327 (89%) of which represent 22 intronless tandem repeats of an ≈153-aa sequence (7).
The sequences of 34 VERL repeats from seven abalone species were obtained by random cloning of PCR products amplified with universal VERL primers. A neighbor-joining tree showed that these 34 repeats had been homogenized by concerted evolution, as indicated by repeats having almost identical sequences within the VERL gene of each species (8). The positions of these repeats in the ordered array of VERL repeats comprising one VERL molecule were unknown. Southern analysis confirmed that the majority of the repeat array was homogenized (8). Further analyses of polymorphism and divergence indicated that these VERL repeats did not evolve by positive selection, but were subjected to neutral, or slightly purifying, selection (8, 9).
When the ordered sequence of all 22 VERL repeats of full-length red abalone (H. rufescens) VERL was obtained, it was discovered that the 34 originally cloned repeats represented repeats 3–22. VERL repeats 1 and 2 (numbering from the amino-terminal end) were quite different from repeats 3–22. Phylogenetic analysis showed that these first two repeats had not been subjected to concerted evolution (7). In fact, red abalone VERL repeats 1 and 2 had not homogenized with repeats 3–22 since the divergence of the seven California species (7). The differences between red abalone VERL repeats 1 and 2, compared with repeats 3–22, compelled us to sequence VERL repeats 1–3 from seven other abalone species (six from California and one from Japan).
Materials and Methods
Cloning VERL Repeats 1–3.
Because full-length VERL has no introns within the array of 22 repeats, repeats 1–3 were amplified from purified genomic DNA of the seven abalone species by using standard PCR methods (7). A sense primer 5′ to repeat 1 (5′-GATACCCCAGACCCCAGAGTG-3′) and an antisense primer (5′-TTGGCTGGAATGCTCTC-3′) within the 5′ portion of repeat 4 were based on the full-length red abalone VERL sequence (GenBank accession no. AF453553). (Two other primers occasionally used were sense 5′-CCAGAGAGTTGTCTTTCAAGT-3′ and antisense 5′-GGTTACAAGACG ACTGAT-3′.) PCR products were cloned into pCR 2.1-TOPO and sequenced by using vector primers (TOPO TA Cloning Kit, Invitrogen). GenBank accession numbers for VERL repeats 1–3 are AF490760 (white abalone, Haliotis sorenseni), AF490761 (pinto abalone, Haliotis kamtschatkana), AF490762 (flat abalone, Haliotis walallensis), AF490763 (Japanese abalone, Haliotis discus), AF490764 (pink abalone, Haliotis corrugata), AF490765 (black abalone, Haliotis cracherodii), and AF490766 (green abalone, Haliotis fulgens).
Analysis of VERL Repeats 1 and 2.
Nucleotide sequences were analyzed by using macvector (Accelrys, San Diego). Sequences were aligned by eye, based on the amino acid sequence. The amino acid alignments used are shown in Fig. 3, which is published as supporting information on the PNAS web site, www.pnas.org. The 34 sequences representing repeats 3–22, used in the phylogenetic branching order shown in Fig. 1, were taken from previous work (8, 9). The phylogeny was estimated by using the phylip program dnaml (10), with 500 bootstrap replicates. Likelihood ratio tests were used to determine whether any codon positions were subjected to positive Darwinian selection as indicated by a dN/dS (amino acid replacement changes/amino acid silent changes) ratio >1 (2, 11, 12). The neutral model (M7) was compared with dN/dS ratios estimated according to a β-distribution limited to the interval (0, 1), to a selection model (M8) with an additional class of codons with a dN/dS ratio estimated from the data (where dN/dS can be >1 or <1). The negative of twice the log likelihood difference between the nested models was compared with the χ2-distribution with two degrees of freedom (2, 11, 12). All analyses were checked for convergence by performing the analysis with different starting parameters, particularly with dN/dS ratios of 0.5 and 1.5. An empirical Bayes approach was used to determine sites subjected to positive selection (2, 11, 12). All calculations were performed by using the program paml (12).
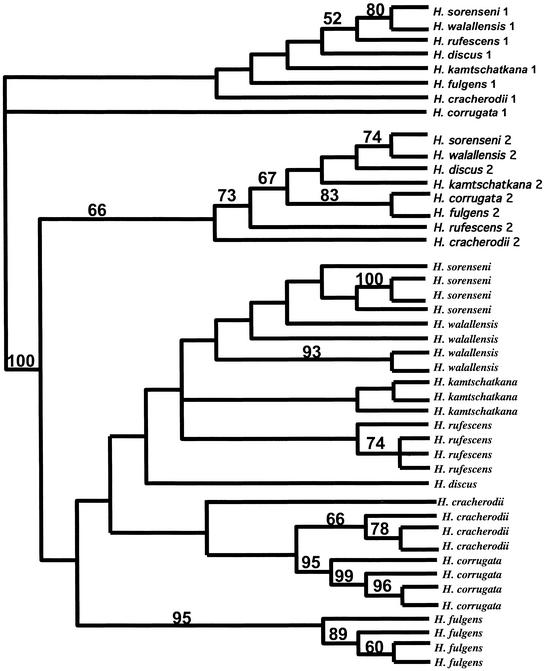
Figure 1.
Maximum likelihood phylogenetic tree of VERL repeats. Bootstrap values (500 replicates) >50% are shown above branches. Repeats 1 and 2 parse on the basis of repeat number, indicating they are not subjected to concerted evolution. However, repeats 3–22 (not numbered) group together according to species and are hence subjected to concerted evolution.
Results and Discussion
Phylogenetic analysis indicates that VERL repeats 1 and 2 have not been subjected to concerted evolution (Fig. 1). For repeats 1 and 2, all eight species group together and show similar species phylogenetic branching relationships, as do sperm lysin (5), mitochondrial CO1 (13), and the internal transcribed spacer of abalone RNA genes (14). In contrast to this parsing, repeats 3–22 group on the basis of species, with paralogous repeats within a gene being more similar than orthologous repeats between species. This indicates that repeats 3–22 have been homogenized by unequal crossing over and/or gene conversion, the process referred to as concerted evolution (8, 9). Thus, repeats 1 and 2 of the VERL repeat array evolve independently of repeats 3–22. The observation that repeats at the end of repeat arrays can evolve independently has been found in other repetitive genes and may arise from the lack of homogenization at the end of the array (15).
Positive selection was tested for by comparing the number of nonsynonymous substitutions per nonsynonymous site (dN) to the number of synonymous substitutions per synonymous site (dS). Because these numbers are normalized to the number of sites, if selection were neutral (i.e., as for a pseudogene) the dN/dS ratio would be equal to 1. An unequivocal sign of positive selection is a dN/dS ratio significantly exceeding 1, indicating a functional benefit to diversify the amino acid sequence (2). Pairwise comparisons of dN/dS for the entire VERL repeats 1 and 2 did not show signs of positive selection (Table 1), with an average ratio of 0.58 (none were >1). This is greater than the average ratio of 0.39 for repeats 3–22. It is possible that only a few sites may be subjected to positive selection and that averaging across all sites obscures the signal of selection. This situation has been observed in other female reproductive proteins that are subjected to positive selection (16).
Table 1.
Parameter estimates for sperm lysin and egg VERL
| Parameter estimated | Lysin | VERL repeats
|
|
|---|---|---|---|
| 1 and 2 | 3–22 | ||
| dN/dS for all sites and lineages | 2.7 | 0.58 | 0.39 |
| % for + selected sites (n) | 32.2% (42) | 12% (42) | NA |
| dN/dS for + selected sites | 8.13 | 3.3 | NA |
| Tree length | 2.34 | 0.75 | 1.3 |
NA, not applicable.
Several methods have recently been developed that allow the detection of variation of the dN/dS ratio between sites (11, 12, 17). Here we have used a maximum likelihood method (11, 12), because the other method (17) requires many more sequences than are currently available for VERL. Power analyses indicate that maximum likelihood methods (11, 12) obtain complete power with tree lengths (substitutions per codon) of ≈1 (18). The tree length provides an indication of the total amount of sequence divergence between all taxa used in the analysis, with larger values indicating greater divergence. The current data sets of lysin, VERL repeats 1and 2, and VERL repeats 3–22 should contain sufficient divergence information for the maximum likelihood methods to detect positive selection as indicated by the tree length data (Table 1).
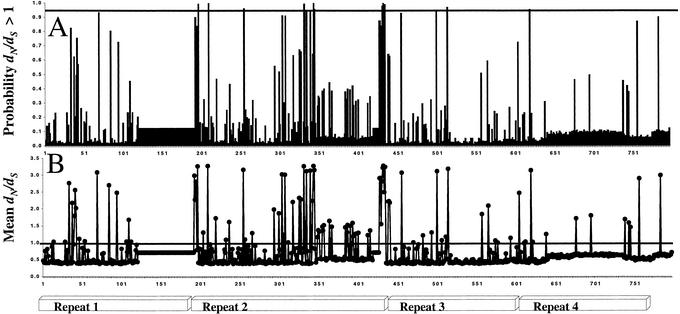
Likelihood ratio tests were used to determine whether any codon positions were subjected to positive Darwinian selection, as indicated by a dN/dS > 1 (11, 12). A neutral model of codon evolution with dN/dS ratios constrained to the interval (0, 1) was compared with a selection model with an additional class of codons with a dN/dS ratio estimated from the data (where dN/dS can be >1 or <1). Analysis by maximum likelihood methods shows that, like sperm lysin, egg VERL repeats 1 and 2 are subjected to positive selection. This is in contrast to VERL repeats 3–22 (8, 9), in which there is no sign of positive selection (Table 1). The selection model was found to fit the data significantly better than the neutral model, indicating that 12% of the sites in VERL repeats 1 and 2 were predicted to be subjected to positive Darwinian selection with a dN/dS ratio of 3.3 (P < 0.01). Of the sites within VERL repeats 1 and 2 predicted to be subjected to positive selection with posterior probabilities >0.95, 82% fall in VERL repeat 2 (Fig. 2). Of these sites, 40% involve changes in the amino acid charge. Changes in charge are consistent with the proposed mechanism of lysin-mediated VE dissolution by the use of positively charged tracts that competitively displace hydrogen bonds between VERL fibers (3). Although the percentage of sites subjected to positive selection is different between lysin and VERL repeats 1 and 2, the total number of sites predicted to be subjected to positive selection (dN/dS > 1) are identical (Table 1). However, it should be noted that the accuracy in prediction of sites subjected to selection depends on the number of sequences available and the total amount of divergence in the data (19). Lysin is more divergent between species than VERL, as shown by the increased tree length.
Figure 2.
Sites predicted by maximum likelihood to be under positive selection. (A) Posterior probabilities of each site being subjected to positive selection. The horizontal line indicates the posterior probability of 0.95. (B) Mean dN/dS ratio for each site. The flat region between 120 and 200 represents an insertion of 71 aa found only in H. corrugata. Rectangles at the bottom show the position of repeats.
Our previous analysis of VERL showed that repeats 3–22 are subjected to concerted evolution and evolve neutrally (8, 9). Because the genomic structure of VERL was not known at that time, this previous work used primers that amplify the main array of repeats 3–22, but not repeats 1 and 2. Having the full-length sequence of VERL, with the repeats ordered by sequencing nested deletions of the total repeat array, showed that repeats 1 and 2 were significantly divergent from repeats 3–22 (7). That original analysis, coupled with the current report, shows that different parts of the VERL repeat array evolve by different mechanisms. Repeats 1 and 2 are the most divergent and are subjected to positive Darwinian selection, whereas repeats 3–22 evolve neutrally and are homogenized by concerted evolution (8, 9). A survey of polymorphism on the C-terminal end of VERL did not detect positive selection (9). The signal of positive selection may have been lost because of recombination between N- and C-terminal coding regions that are separated by almost 10 kb and may have high levels of recombination, as shown by the presence of concerted evolution. Recombination would unlink neutrally evolving sites from the gene region subjected to positive selection, preventing the detection of selection by comparison of the level and frequency of polymorphic sites.
Binding data indicate that approximately two lysins bind to each VERL repeat, meaning that ≈44 lysins can bind to one VERL molecule. Binding is high affinity with a Kd of ≈10−9 M. Species-specific binding was observed by using purified VERL molecules and sperm lysin, although the magnitude was not as great as lysin-mediated dissolution of intact VEs (6). Because purified VERL molecules contain mainly repeats 3–22, this indicates that lysin may have two targets that regulate specificity. Repeats 1 and 2 could regulate the majority of species specificity of lysin binding, whereas repeats 3–22 could recognize lysin with less specificity. The kinetics of VE dissolution by lysin show that the greatest species specificity is in the initiation of the VE dissolution reaction (6, 20). However, it is likely that lysin has to adapt to the neutrally drifting array of repeats 3–22 (8, 9) because species-specific binding was observed with whole VERL molecules (6). It will be of interest to measure binding affinities between lysin and individually expressed repeats. The existence in VERL of two targets subject to different evolutionary forces to which lysin adapts could explain the extraordinarily rapid divergence observed in abalone sperm lysin (3–5, 13, 20).
Sperm lysin and egg VERL are a cognate pair of gamete recognition proteins that regulate fertilization in abalone. Although gamete recognition proteins are known to exist in both male and female gametes of various species, the abalone provides the only system in which high-affinity binding has been demonstrated in a cognate pair of gamete recognition proteins. The fact that both VERL and lysin sequences are known from several congeneric species, permits a molecular evolutionary analysis (3–9). The discovery that positive Darwinian selection acts on both lysin and VERL leads to the question of what the selective force(s) could be that drive this adaptive change. One protein might be adapting to changes in the other, but which leads the rapid coevolutionary chase? Two hypotheses are discussed below: sexual selection by eggs (cryptic female choice) and sexual conflict. Both hypotheses have overlapping predictions, and alternative hypotheses such as the “microbial attack on eggs” (20) could also be invoked.
The sexual selection hypothesis is based on the idea that certain eggs interact more successfully with sperm carrying novel genotypes of the cognate binding partner. Thus, within the population, there are eggs and sperm that have sequence variations in interacting surface proteins with a range of affinities for binding each other. Evidence has been presented that sea urchin eggs prefer to be fertilized by sperm with the same genotype of sperm binding protein that they possess (21). Similar findings have also been presented for Coleoptera (22). Experiments on Drosophila hybridization also provide evidence for the rapid coevolution of the gamete recognition system by sexual selection (23). Mathematical simulations show that sexual selection acting on gamete recognition proteins could account for their rapid divergence that could result in sympatric speciation (24); however, these models require ecological competition that may not be necessary for isolation by divergence of reproductive proteins.
The theory of sexual conflict has been of major interest to both theoretical (25, 26) and experimental evolutionary biologists (27, 28). In sexual conflict, males and females have different interests in reproduction; selection favors traits in one sex that increase the fitness of one sex at the cost of lowered fitness in the opposite sex. For example, it might be advantageous for females to evolve lower receptivity to males, whereas males would be constantly evolving to catch females who are evolving away from them (26, 28). Over time, sexual conflict could result in the sympatric splitting of one population into two new species (29, 30). Sexual conflict has been shown to be “a key engine of speciation” in insects (31). Although many papers have dealt with the theory of sexual conflict in speciation, there are to date no examples of the coevolution of interacting male and female reproductive proteins driven by sexual conflict and positive selection (25). Mathematical models show that sexual conflict at the level of gamete recognition proteins could lead to sympatric speciation (25, 26). For example, based on sexual conflict, eggs may evolve ways to slow sperm from fusing with them to avoid polyspermic fertilization, which results in the arrest of development (32). However, sperm competition (33, 34) constantly selects for sperm that are more efficient at fusing with eggs. Mathematical models show that sexual conflict can result in continuous evolution of female and female reproductive characters. Genes involved in this “coevolutionary chase” of female and male interacting traits are predicted to show rapid evolution and positive Darwinian selection (24–29, 35). The analysis of our sequence data showing positive selection in both VERL and lysin is in excellent agreement with these mathematical models.
Mathematical models demonstrate that sexual conflict can lead to a variety of outcomes in addition to an endless coevolutionary chase (26). For example, the models indicate that females may split into two groups with different genotypes. Under some circumstances, this will “trap” males in a state where they are unable to adapt to either genotype and be forced to take a middle ground. Our current and previous data are consistent with these mathematical models. Previous results have demonstrated that lysin of two species shows the signature of a selective sweep (9, 13). Interestingly, egg VERL from the pink abalone (H. corrugata) shows two distinct genotypes (9). It will be of interest to see whether the sperm protein lysin in this population is fixed at a middle state or has also diversified and adapted to each VERL type, perhaps indicating an incipient sympatric speciation event driven by the rapid coevolution of reproductive proteins.
The observation of diverse reproductive molecule genotypes within a species is not limited to abalone. It is interesting to note that sea urchins exhibit two or more distinct reproductive loci genotypes in several genera. Echinometra mathaei sea urchins have two genotypes of the sperm adhesive protein bindin. Eggs of this species show a statistically significant preference to be fertilized by sperm carrying their same bindin genotype (21). Sea urchin eggs are covered by a jelly layer containing a fucose sulfate polymer (FSP) that binds to sperm receptors to induce the acrosome reaction (36). In the San Diego population of the species Strongylocentrotus purpuratus, 64% of females have eggs with one form of the polymer, whereas 31% of females have eggs with the other isotype of FSP. Only 5% of females have eggs with both types of polymer (n = 136; V.D.V., unpublished data). Each type of FSP polymer is equally potent at inducing the sperm acrosome reaction (36). Two female-distinct FSPs are also synthesized by females of the sea urchin S. drobachiensis; however, all other species of sea urchins thus far examined have only one form of FSP (37). These examples show that the differentiation of gamete recognition molecules within extant species of broadcast spawning marine invertebrates could be happening at this time. Such preliminary data supports the idea that differentiation of gamete recognition systems could result in sympatric speciation.
Supplementary Material
Acknowledgments
We thank Dr. J. D. Calkins for helpful discussions and two anonymous reviewers for comments. This work was supported by National Institutes of Health Grant HD12986 (to V.D.V.) and National Science Foundation Grant DEB-0111613 (to W.J.S.).
Abbreviations
- VE
vitelline envelope
- VERL
VE receptor of lysin
Footnotes
References
- 1.Swanson W J, Vacquier V D. Nat Rev Genet. 2002;3:137–144. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- 2.Yang Z, Bielawski J P. Trends Ecol Evol. 2000;15:496–503. doi: 10.1016/S0169-5347(00)01994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kresge N, Vacquier V D, Stout C D. BioEssays. 2001;23:95–103. doi: 10.1002/1521-1878(200101)23:1<95::AID-BIES1012>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Swanson W J, Vacquier V D. Mol Biol Evol. 2000;17:1446–1455. doi: 10.1093/oxfordjournals.molbev.a026245. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y-H, Ota T, Vacquier V D. Mol Biol Evol. 1995;12:231–238. doi: 10.1093/oxfordjournals.molbev.a040200. [DOI] [PubMed] [Google Scholar]
- 6.Swanson W J, Vacquier V D. Proc Natl Acad Sci USA. 1997;94:6724–6729. doi: 10.1073/pnas.94.13.6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galindo B E, Moy G W, Swanson W J, Vacquier V D. Gene. 2002;288:111–117. doi: 10.1016/s0378-1119(02)00459-6. [DOI] [PubMed] [Google Scholar]
- 8.Swanson W J, Vacquier V D. Science. 1998;281:710–712. doi: 10.1126/science.281.5377.710. [DOI] [PubMed] [Google Scholar]
- 9.Swanson W J, Aquadro C F, Vacquier V D. Mol Biol Evol. 2001;18:376–383. doi: 10.1093/oxfordjournals.molbev.a003813. [DOI] [PubMed] [Google Scholar]
- 10.Felsenstein J. phylip (Phylogeny Inference Package) (Univ. of Washington, Seattle), Version 3.6. 1993. [Google Scholar]
- 11.Nielsen R, Yang Z. Genetics. 1998;148:929–936. doi: 10.1093/genetics/148.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z, Nielsen R, Goldman N, Pedersen A M. Genetics. 2000;155:431–449. doi: 10.1093/genetics/155.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metz E C, Robles-Sikisaka R, Vacquier V D. Proc Natl Acad Sci USA. 1998;95:10676–10681. doi: 10.1073/pnas.95.18.10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman A W, Vacquier V D. J Mol Evol. 2002;54:246–257. doi: 10.1007/s00239-001-0006-0. [DOI] [PubMed] [Google Scholar]
- 15.McAllister B F, Werren J H. J Mol Evol. 1999;48:469–481. doi: 10.1007/pl00006491. [DOI] [PubMed] [Google Scholar]
- 16.Swanson W J, Yang Z, Wolfner M F, Aquadro C F. Proc Natl Acad Sci USA. 2001;98:2509–2014. doi: 10.1073/pnas.051605998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki Y, Gojobori T. Mol Biol Evol. 1999;16:1315–1328. doi: 10.1093/oxfordjournals.molbev.a026042. [DOI] [PubMed] [Google Scholar]
- 18.Anisimova M, Bielawski J P, Yang Z. Mol Biol Evol. 2001;18:1585–1592. doi: 10.1093/oxfordjournals.molbev.a003945. [DOI] [PubMed] [Google Scholar]
- 19.Anisimova M, Bielawski J P, Yang Z. Mol Biol Evol. 2002;19:950–958. doi: 10.1093/oxfordjournals.molbev.a004152. [DOI] [PubMed] [Google Scholar]
- 20.Vacquier V D, Lee Y-H. Zygote. 1993;1:181–196. doi: 10.1017/s0967199400001465. [DOI] [PubMed] [Google Scholar]
- 21.Palumbi S R. Proc Natl Acad Sci USA. 1999;96:12632–12637. doi: 10.1073/pnas.96.22.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown D V, Eady P E. Evolution (Lawrence, Kans) 2001;55:2257–2262. doi: 10.1111/j.0014-3820.2001.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 23.Blows M W. Proc R Soc London B. 1999;266:2169–2174. doi: 10.1098/rspb.1999.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Doorn G S, Luttikhuizen P C, Weissing F J. Proc R Soc London B. 2001;268:2155–2161. doi: 10.1098/rspb.2001.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavrilets S. Nature. 2000;403:886–889. doi: 10.1038/35002564. [DOI] [PubMed] [Google Scholar]
- 26.Gavrilets S, Waxman D. Proc Natl Acad Sci USA. 2002;99:10533–10538. doi: 10.1073/pnas.152011499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice W R. Nature. 1996;381:232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- 28.Rice W R, Holland B. Behav Ecol Sociobiol. 1997;41:1–10. [Google Scholar]
- 29.Gavrilets S, Arnqvist G, Friberg U. Proc R Soc London B. 2001;268:531–539. doi: 10.1098/rspb.2000.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker G A, Partridge L. Philos Trans R Soc London B. 1998;353:261–274. doi: 10.1098/rstb.1998.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnqvist G, Edvardsson M, Friberg U, Nilsson T. Proc Natl Acad Sci USA. 2000;97:10460–10464. doi: 10.1073/pnas.97.19.10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frank S A. Evol Ecol Res. 2000;2:613–625. [Google Scholar]
- 33.Clark A G, Begun D J, Prout T. Science. 1999;283:217–220. doi: 10.1126/science.283.5399.217. [DOI] [PubMed] [Google Scholar]
- 34.Birkhead T R, Pizzari T. Nat Rev Genet. 2002;3:262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- 35.Howard D J. Annu Rev Ecol Syst. 1999;30:109–132. [Google Scholar]
- 36.Vacquier V D, Moy G W. Dev Biol. 1997;192:125–135. doi: 10.1006/dbio.1997.8729. [DOI] [PubMed] [Google Scholar]
- 37.Vilela-Silva A C E S, Castro M O, Valente A P, Biermann C H, Mourao P A S. J Biol Chem. 2002;277:379–387. doi: 10.1074/jbc.M108496200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.