Abstract
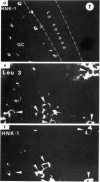
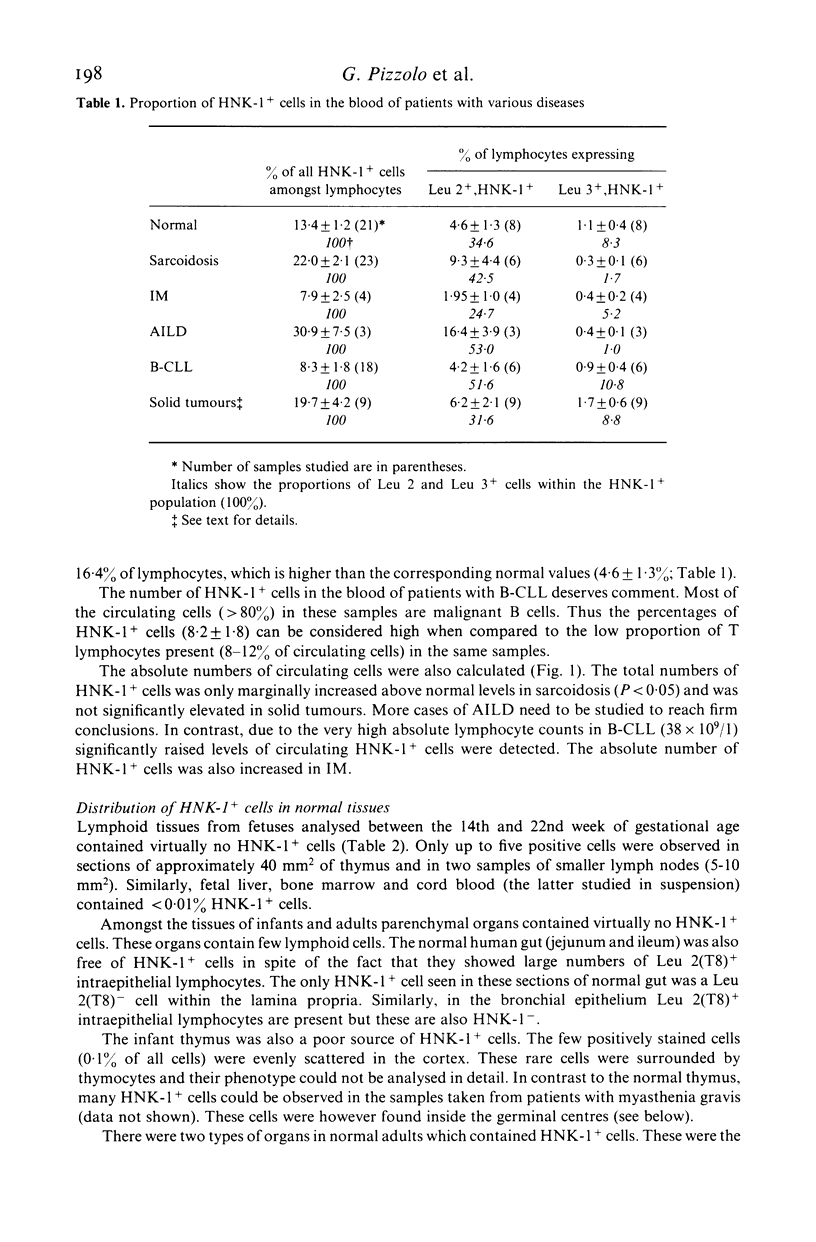
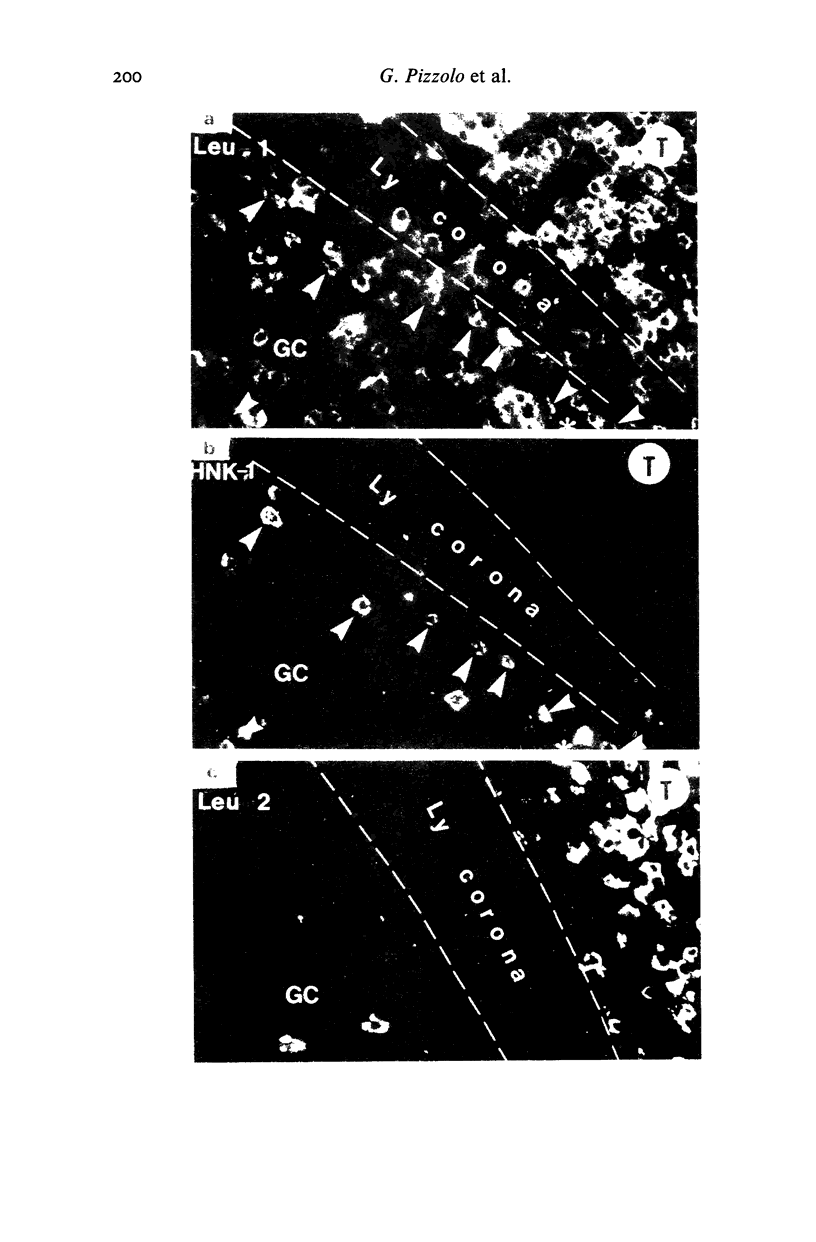
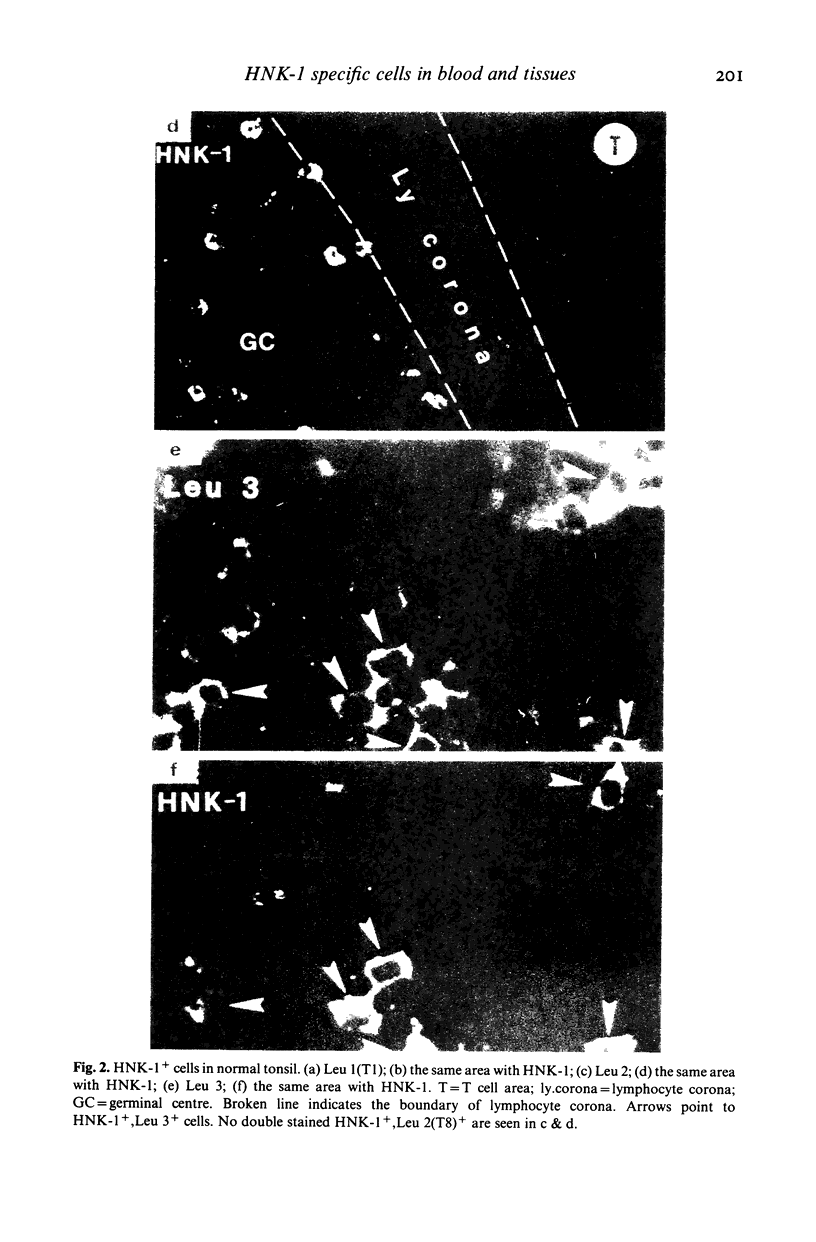
When studied with double staining techniques HNK-1+ cells include subsets not expressing T cell antigens (A), expressing T8 antigens (B) and expressing T4 antigens (C). Cells with phenotype A are observed as the dominant HNK-1+ population (greater than 50% of all HNK-1+ cells) in the blood from controls and from patients with solid tumours, infectious mononucleosis and sarcoidosis. Cells with phenotype B are always a substantial subset (35% of HNK-1+ cells) in the peripheral blood but in patients with B chronic lymphocytic leukaemia and angioimmunoblastic lymphadenopathy these cells are present in an even higher percentage (greater than 50% of all HNK-1+ cells). This cell subset is the only HNK-1+ population found in the few tumour samples where HNK-1+ cells are identifiable. Apart from these few cases of malignancies, the type A and B subsets are rare in the tissues. In these samples Leu 11+ cells seem to be absent. In contrast, cells with phenotype C are a minor population in the blood but represent most HNK-1+ cells in the germinal centres of lymph nodes and their malignant counterparts in follicular centre cell lymphoma. These HNK-1+, T4+ cells are Leu 11-. These phenotypic characteristics indicate that the most efficient NK cells may represent a circulating and not a tissue seeking population.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. I. Two distinct phenotypes of human NK cells with different cytotoxic capability. J Immunol. 1982 Oct;129(4):1752–1757. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Postnatal expansion of the natural killer and keller cell population in humans identified by the monoclonal HNK-1 antibody. J Exp Med. 1982 Jan 1;155(1):321–326. doi: 10.1084/jem.155.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo T., Miller C. A., Gartland G. L., Balch C. M. Differentiation stages of human natural killer cells in lymphoid tissues from fetal to adult life. J Exp Med. 1983 Jan 1;157(1):273–284. doi: 10.1084/jem.157.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson K., Hansen J. A., Storb R., Goehle S., Goldstein G., Thomas E. D. T-cell subpopulations identified by monoclonal antibodies after human marrow transplantation. I. Helper-inducer and cytotoxic-suppressor subsets. Blood. 1982 Jun;59(6):1292–1298. [PubMed] [Google Scholar]
- Chilosi M., Pizzolo G., Vincenzi C. Haematoxylin counterstaining of immunofluorescence preparations. J Clin Pathol. 1983 Jan;36(1):114–115. doi: 10.1136/jcp.36.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrot M., Janossy G., Tidman N., Blacklock H., Lopez E., Bofill M., Lampert I., Morgenstein G., Powles R., Prentice H. G. T cell regeneration after allogeneic bone marrow transplantation. Clin Exp Immunol. 1983 Oct;54(1):59–72. [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B. Natural killer (NK) cells and their possible roles in resistance against disease. Clin Immunol Rev. 1981;1(1):1–65. [PubMed] [Google Scholar]
- Hiserodt J. C., Britvan L. J., Targan S. R. Inhibition of human natural killing by heterologous and monoclonal antibodies. J Immunol. 1982 Nov;129(5):2248–2254. [PubMed] [Google Scholar]
- Kiessling R., Wigzell H. An analysis of the murine NK cell as to structure, function and biological relevance. Immunol Rev. 1979;44:165–208. doi: 10.1111/j.1600-065x.1979.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Kimber I., Moore M. Naturally cytotoxic tonsillar lymphocytes: a manifestation of heterogeneity among human NK cells. Scand J Immunol. 1983 Jan;17(1):29–36. doi: 10.1111/j.1365-3083.1983.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Nieminen P., Paasivuo R., Saksela E. Effect of a monoclonal anti-large granular lymphocyte antibody on the human NK activity. J Immunol. 1982 Mar;128(3):1097–1101. [PubMed] [Google Scholar]
- Oi V. T., Glazer A. N., Stryer L. Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. J Cell Biol. 1982 Jun;93(3):981–986. doi: 10.1083/jcb.93.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortaldo J. R., Sharrow S. O., Timonen T., Herberman R. B. Determination of surface antigens on highly purified human NK cells by flow cytometry with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2401–2409. [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Pizzolo G., Chilosi M., Cetto G. L., Fiore-Donati L., Janossy G. Immuno-histological analysis of bone marrow involvement in lymphoproliferative disorders. Br J Haematol. 1982 Jan;50(1):95–100. doi: 10.1111/j.1365-2141.1982.tb01894.x. [DOI] [PubMed] [Google Scholar]
- Potter M. R., Moore M. Organ distribution of natural cytotoxicity in the rat. Clin Exp Immunol. 1978 Oct;34(1):78–86. [PMC free article] [PubMed] [Google Scholar]
- Powles R. L., Clink H. M., Spence D., Morgenstern G., Watson J. G., Selby P. J., Woods M., Barrett A., Jameson B., Sloane J. Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet. 1980 Feb 16;1(8164):327–329. doi: 10.1016/s0140-6736(80)90881-8. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Ritchie A. W., James K., Micklem H. S. The distribution and possible significance of cells identified in human lymphoid tissue by the monoclonal antibody HNK-1. Clin Exp Immunol. 1983 Mar;51(3):439–447. [PMC free article] [PubMed] [Google Scholar]
- Rumpold H., Kraft D., Obexer G., Böck G., Gebhart W. A monoclonal antibody against a surface antigen shared by human large granular lymphocytes and granulocytes. J Immunol. 1982 Oct;129(4):1458–1464. [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Goldstein G., Jewell D. P. T lymphocyte subsets in human intestinal mucosa: the distribution and relationship to MHC-derived antigens. Clin Exp Immunol. 1981 Jun;44(3):453–458. [PMC free article] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Mason D. Y. The normal and malignant germinal centre. Clin Haematol. 1982 Oct;11(3):531–559. [PubMed] [Google Scholar]
- Talmadge J. E., Meyers K. M., Prieur D. J., Starkey J. R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980 Apr 17;284(5757):622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Tilden A. B., Abo T., Balch C. M. Suppressor cell function of human granular lymphocytes identified by the HNK-1 (Leu 7) monoclonal antibody. J Immunol. 1983 Mar;130(3):1171–1175. [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. F., Dennert G. Effects of a cloned cell line with NK activity on bone marrow transplants, tumour development and metastasis in vivo. Nature. 1982 Nov 4;300(5887):31–34. doi: 10.1038/300031a0. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Clouse K. A., Biddison W. E., Kung P. C. Phenotypes of human natural killer cell populations detected with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2575–2580. [PubMed] [Google Scholar]